Article Text
Abstract
Aims: To compare functional and anatomical outcomes of intravitreal bevacizumab (Avastin) and verteporfin (photodynamic) therapy (PDT) combined with intravitreal triamcinolone (IVTA) in patients with neovascular age-related macular degeneration (AMD).
Methods: Twenty-eight patients with neovascular AMD were enrolled in a prospective, randomised, controlled clinical trial. All patients randomly assigned to 1 mg intravitreal bevacizumab (0.04 ml) received three initial treatments at 4-week intervals. In further follow-up retreatment was based on optical coherence tomography (OCT). Patients randomly assigned to standard PDT received a same-day intravitreal injection of 4 mg triamcinolone (Kenalog). Retreatment was based on fluorescein angiography at 3-month intervals. Functional and anatomical results were evaluated using the Early Treatment Diabetic Retinopathy Study protocol vision charts, fluorescein angiography and OCT.
Results: In the bevacizumab-treated group mean visual acuity (VA) improved to a 2.2 line gain at 6 months follow-up. Eyes treated in the PDT plus IVTA group had a stable mean VA at month 6 compared with baseline. There was a statistically significant difference (p = 0.03, analysis of variance (ANOVA)) between both groups as early as one day after initial treatment. The reduction in central retinal thickness (CRT) showed no significant difference between both groups (p = 0.3, ANOVA). Mean CRT was reduced from 357 μm at baseline to 239 μm at month 6 in bevacizumab-treated patients and from 326 μm to 222 μm, respectively, in PDT plus IVTA-treated patients. No significant local or systemic safety concerns were detected up to month 6.
Conclusion: Intravitreal bevacizumab showed promising 6-month results in patients with neovascular AMD. Functional outcomes appear not only to be dependent on a reduction in CRT but also on the treatment modality used.
Statistics from Altmetric.com
Anti-vascular endothelial growth factor (VEGF) therapy for age-related macular degeneration (AMD), one of the leading causes of severe vision loss in the developed world,1–3 has probably been the most promising breakthrough in medical retina treatment in recent years. For the first time in the treatment of neovascular AMD a therapeutic strategy binding all isoforms of VEGF has been able to show a real stabilisation of vision in phase III clinical trials.4 5 An improvement in vision has even been reported in approximately 25% to 40% of patients at 1-year follow-up and appears to be maintained at 2 years. These results have, however, been obtained using monthly re-injections of ranibizumab independent of disease status. A recent prospective study on an optical coherence tomography (OCT)-based retreatment regimen for ranibizumab obtained similar results with regard to maintaining and improving vision. This treatment regimen also allowed a reduction in the number of treatments to 5.6 within the first year but did not reduce the number of patient visits.6
Bevacizumab, a full-length monoclonal antibody binding all isoforms of VEGF, has been primarily developed for systemic therapy in cancer patients. The first reports on “off-label” bevacizumab in ophthalmology showed the systemic use of the drug to be effective in patients with neovascular AMD.7 8 The real breakthrough for the use of bevacizumab came with first reports on the effectiveness of “off-label” intravitreal bevacizumab for neovascular AMD and retinal vein occlusions,9 10 as well as studies documenting the retinal penetration of the relatively large (∼150 kDa) monoclonal antibody.11 In the meantime, several retrospective and prospective studies have indicated beneficial effects and a good safety profile of intravitreal bevacizumab for neovascular AMD, but phase III studies are currently missing.12–20 A major disadvantage of the inexpensive “off-label” drug appears to be, similar to other intravitreally given anti-VEGF drugs, the frequent requirement for retreatment.
The combination of verteporfin (photodynamic) therapy (PDT) with intravitreal triamcinolone (IVTA) has been the standard of care for many patients with neovascular AMD. Major advantages of this combination strategy appeared to be better functional results compared with PDT or IVTA monotherapy and an extended treatment durability requiring retreatment as infrequently as less than two treatments per year.21–24 The major disadvantages were steroid-induced adverse effects such as ocular hypertension/glaucoma and the progression of cataracts.25 26
The present study compares functional and anatomical outcomes of 1 mg (0.04 ml) intravitreal bevacizumab with PDT plus 4 mg IVTA (0.1 ml).
PATIENTS AND METHODS
The study protocol was approved by the Ethics Committee of the Medical University of Vienna, the Austrian health authorities, was registered at the European Clinical Trials Database (EudraCT no 2005-003288-21) and adhered to the guidelines of the declaration of Helsinki. All patients signed written informed consent before enrollment into the study.
The study was designed as an open-label, single-centre, randomised, controlled clinical trial. Primary and secondary outcomes were a change in mean visual acuity (VA) and mean 1 mm central retinal thickness comparing both treatment groups.
Enrolled patients had neovascular AMD of any lesion type smaller than four disc areas, without any previous treatment for neovascular AMD, and a VA of 20/40 to 20/800. Patients with a history of thromboembolic events within the past 3 months and a predictable need for ocular surgery were excluded from the study. Patients were randomly assigned 1 : 1 to 1 mg intravitreal bevacizumab or standard PDT (6 mg/m2, 50 J/cm2, 600 mW/cm2, 83 seconds light application) plus 4 mg IVTA.
All patients were evaluated at baseline, day 1, day 7, months 1, 3 and 6 by VA testing using Early Treatment Diabetic Retinopathy Study (ETDRS) charts at two meters, OCT and complete ophthalmic examination. Fluorescein angiography and indocyanine green angiography as well as microperimetry were performed at baseline, months 3 and 6. Patients randomly assigned to intravitreal bevacizumab were also seen at months 2, 4 and 5.
Three initial injections of 1 mg (0.04 ml) bevacizumab were given at monthly intervals up to month 2, thereafter retreatment was based on findings of OCT only. Indication for retreatment after the third injection was evidence of persistent or recurrent intra or subretinal fluid by OCT. A stable pigment epithelial detachment without these findings was no indication for retreatment. Patients in the PDT plus IVTA group were retreated at 3-month intervals if there was evidence of leakage by fluorescein angiography.
Intravitreal injections of bevacizumab (Avastin; Roche Basel, CH; Genentech, Inc, South San Francisco, California, USA) and triamcinolone acetonide (Volon A; Bristol-Myers Squibb, New York, USA) were both performed in the operating room under sterile conditions following the guidelines of the Austrian ophthalmological society. For patients with previous PDT operating room lights were dimmed to avoid further photoactivation of verteporfin.
Bevacizumab was prepared by the institutional pharmacy as sterile filled and packed tuberculin syringes containing 0.2 ml; 0.04 ml (1 mg) bevacizumab was injected intravitreally using a 30 gauge needle. Triamcinolone acetonide was washed and prepared according to previous publications.27 A 27 gauge needle was used to inject 4 mg triamcinolone (0.1 ml) intravitreally.
For statistical analysis analysis of variance (ANOVA) was used to compare mean VA and mean CRT of both treatment arms. For comparison of VA and CRT outcomes within each group with baseline the t-test was used. P values of less than 0.05 were considered statistically significant.
RESULTS
Twenty-eight patients, 19 women and nine men, were enrolled into the study, 14 into each arm. The mean age was 78 (SD 8) years (range 58 to 88 years). Baseline characteristics were well balanced (table 1). There was no statistically significant difference with regard to VA and CRT at baseline.
Up to month 6 no patient was excluded from the study and month 6 follow-up was 100% in the bevacizumab-treated group and one patient failed to follow up at month 6 in the PDT plus IVTA-treated group.
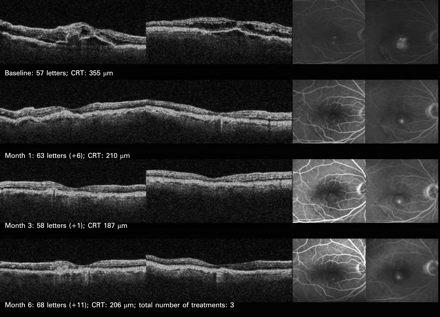
Mean VA of eyes enrolled into the bevacizumab treatment arm improved from 50 letters (20/100) at baseline to 54 letters (20/80−1) at day 1, 58 letters (20/63−2) at week 1, 62 letters (20/63+2) at month 3 and 61 letters (20/63+1) at month 6 (fig 1). Compared with baseline changes in VA were statistically significant (p = 0.005, t-test) as early as week 1.
Mean VA of eyes enrolled into the PDT plus IVTA group showed a moderate decrease from 46 letters (20/125+1) at baseline to 40 letters (20/160) at day 1 and then returned to 46 letters (20/125+1) at week 1, stayed at 46 letters (20/125+1) at month 3 and 46 letters (20/125+1) at month 6 (fig 1).
Comparing the mean VA of both groups, the primary endpoint for the study, showed a statistically significant difference between both groups in favour of the bevacizumab-treated group as early as day 1 (p = 0.03, ANOVA between groups). Table 2 outlines the distribution of change in VA for both groups.
Mean CRT showed similar changes for both groups up to month 6 follow-up. It decreased in the bevacizumab-treated group from 357 μm at baseline to 342 μm at day 1, 268 μm at week 1, 230 μm at month 3 and 239 μm at month 6 (fig 2). Compared with baseline the decrease in mean CRT became statistically significant as early as week 1 (p<0.005). In the PDT plus IVTA group mean CRT decreased from 326 μm at baseline, except for an increase to 387 μm at day 1, continuously to 243 μm at week 1, 234 μm at month 3 and 222 μm at month 6 (fig 2). Compared with baseline the decrease in CRT became statistically significant as early as week 1 (p = 0.002). There was no statistically significant difference between both groups with regard to CRT (p = 0.3, ANOVA between groups), the secondary endpoint of the study, even not on day 1 (p = 0.2, t-test). Figures 3 and 4 show examples of eyes treated in both treatment arms.
The mean number of treatments including month 6 was 4.5 out of seven possible treatments in the bevacizumab-treated group and 1.9 out of three possible treatments in the PDT plus IVTA-treated group. At months 3 and 6 50%/43% required retreatment in the bevacizumab group and 79%/14%, respectively, in the PDT plus IVTA group.
The correlation coefficient (r) was used for the calculation of the correlation between the change in VA and a change in CRT. A significant correlation was found for the bevacizumab-treated group at month 1 (r = −0.55; p = 0.04) but not for the PDT plus IVTA-treated group (r = −0.19; p = 0.5). At months 3 and 6 the correlations did not quite reach significance in the bevacizumab-treated group (r = −0.5; p = 0.066 and r = −0.47; p = 0.089, respectively) and were again not significant for the PDT plus IVTA-treated group (r = −0.24; p = 0.4 and r = −0.10; p = 0.7, respectively).
In none of the groups was a severe ocular (eg traumatic cataract, retinal detachment, endopthalmitis, severe ocular inflammation) or systemic adverse event reported up to month 6. Two patients in the PDT plus IVTA group experienced slightly elevated intraocular pressure (IOP) values (25 and 26 mm Hg) during one week after initial treatment and received topical IOP-lowering medication. For none of the bevacizumab-treated patients was an elevated IOP detected. In neither group was significant cataract progression noted within 6 months follow-up. Indocyanine green angiography showed no evidence of significant bevacizumab-associated choroidal perfusion changes. Patients in the PDT plus IVTA group had characteristic hypofluorescence within the area of the PDT treatment spot, but there was no evidence of treatment-associated choroidal hypoperfusion outside the area of the PDT treatment spot.
DISCUSSION
The results of this prospective, randomised, controlled clinical study have shown several clinically relevant findings. The use of 1 mg intravitreal bevacizumab appears to have significantly better visual outcomes than the combination therapy of PDT plus 4 mg IVTA in a selected patient population with neovascular AMD up to 6 months. Patients receiving bevacizumab gained a mean of more than two lines compared with a stable visual outcome in patients treated with PDT plus IVTA. The promising functional and anatomical results go along with results from uncontrolled studies.13–20 No severe ocular or systemic adverse events were found in both study groups up to 6 months. The study was, however, too small to provide profound safety data.
The results further indicate that an intermittent treatment regimen using OCT findings as an indicator for retreatment is most promising for managing AMD patients with intravitreal bevacizumab. Similar results have been published for the use of ranibizumab in neovascular AMD.6 Despite the risk of CNV recurrence and no fixed retreatment at month 5 the study protocol allowed for a significant improvement of vision. The major limitation of this study protocol, especially when compared with the retreatment regimen used for PDT plus IVTA, is that despite reducing the number of injections, the number of patient visits is not reduced.
A correlation of changes in CRT and VA has been found for systemic bevacizumab in neovascular AMD.7 This seems to be valid also for intravitreal bevacizumab at month 1 as shown in this study. This correlation appears to be dependent on the treatment modality used. The PDT plus IVTA-treated patients showed, except for a PDT-induced increase in CRT at day 1, a very similar time course and degree of reduction in CRT compared with bevacizumab-treated patients. However, VA results did not correlate with the CRT reduction. One can only speculate on the reasons for this finding. It is well known that standard PDT induces a transient reduction in the outer blood retinal barrier function early after treatment,28 29 which seems not to be completely inhibited by same-day 4 mg IVTA. PDT furthermore leads to an at least transient closure of the choriocapillaris,28 and an atrophy of the retinal pigment epithelium (RPE) after repeated treatments.30 Modified PDT treatment parameters might (eg reduced fluence) have less effect on choroid and RPE and might subsequently provide better outcomes.31 Triamcinolone, even after washing out preservatives, has also shown a toxic effect on RPE cells in cell culture models.32 Which treatment component is responsible for the worse functional outcomes in the PDT plus IVTA group remains to be determined. These findings might, however, make us further aware of the potential limitations of combination strategies with anti-angiogenic drugs, not with regard to treatment durability, but with regard to functional outcomes.
Despite the prospective, randomised, controlled study design, the study has clear limitations. The study was small, selected patients with relatively small, active CNV without any previous treatment and is so far limited to 6 months follow-up.
The trend indicated by the results of this study emphasises the potential value of intravitreal bevacizumab in neovascular AMD and supports the need for further large controlled clinical trials.
REFERENCES
Footnotes
-
Competing interests: US-E is an owner of the patent on the use of green porphyrins in neovasculature of the eye under the guidelines of the Wellman Laboratories of Photomedicine, Harvard Medical School, Boston, Massachusetts, USA.
Linked Articles
- At a glance