Article Text
Statistics from Altmetric.com
Introduction
Age-related macular degeneration (AMD) is the most common cause for legal blindness in the developed countries. Intravitreal application of anti-VEGF molecules is the gold standard for treatment the exudative form of this disease.1 In the clinic, different VEGF antagonists are currently in use, the approved ranibizumab, the off-label bevacizumab, and the recently approved aflibercept.2–4 For effective treatment, repeated intravitreal injections are needed, and, for ranibizumab, best results were obtained by monthly injections, even though in clinical practice, other treatment regiments are usually followed.5 Additionally, in exudative AMD, the treatment may have to be continued for several years, as the cessation of anti-VEGF treatment may result in the reoccurrence of choriodal neovascularisations, bleeding and vision loss.6
The currently used anti-VEGF molecules, bevacizumab, ranibizumab and aflibercept, differ not only in their VEGF binding affinity, but also in their molecular structure. Bevacizumab is a humanised murine full-length antibody against VEGF-A with a molecular weight of 149 kDa, while ranibizumab is a high affinity Fab-fragment against VEGF-A with a molecular weight of 48 kDa, lacking the Fc-fragment. Aflibercept is a fusion protein with a molecular weight of 115 kDa, composed of the 2nd Ig domain of the VEGFR-1 and the 3rd Ig domain of the VEGFR-2 connected to an Fc-fragment.7 These molecular differences may result in different effects on retinal cells.
We have previously investigated and compared the effect of ranibizumab and bevacizumab on RPE cells, finding apparent differences. Bevacizumab, but not ranibizumab, is taken up by and stored in RPE cells for at least 7 days. The presence of intracellular bevacizumab impairs the phagocytic function of RPE cells.8 ,9 An uptake of bevacizumab by endothelial cells has recently been reported.10
In this study, we investigated the effect of aflibercept on RPE cells, regarding toxicity, wound healing, cellular uptake and phagocytosis.
Material and methods
Primary RPE isolation and culture
Porcine RPE cells were isolated as previously described.11 In brief, porcine eyes obtained from the local abattoir were cleaned of adjacent tissue and immersed briefly in antiseptic solution (betaisodona, Mundipharma, Limburg, Germany). The anterior part of the eye was removed, as well as lens, vitreous and retina. In each eye cup, trypsin was added, and incubated for 10 min at 37 °C. Trypsin solution was removed and substituted with trypsin-EDTA for 45 min at 37 °C. RPE cells were gently pipetted off the choroid, collected in media and washed. Cells were cultivated in Dulbecco's modified Eagle's medium (DMEM) supplemented with penicillin/streptomycin (1%), HEPES (25 mM), sodium-pyruvat (110 mg/mL), and 10% fetal calf serum (Linaris GmbH, Wertheim-Bettingen, Germany).
Treatment of cells
Confluent primary porcine RPE cells of second or third passage were treated with different concentrations of bevacizumab (125 µg/mL, 250 µg/mL), ranibizumab (125 µg/mL) or aflibercept (125 µg/mL, 500 µg/mL) for various time periods (1 h, 4 h, 24 h, 3 days, 5 days, 7 days). Concentration and time frame were dependent on the respective experiment. Medium was changed after the third day of incubation without addition of the respective VEGF inhibitor.
MTT—assay
Cell viability in cell culture after 24 h and 7 days of aflibercept incubation was tested with methyl thiazolyl tetrazolium (MTT) assay as described elsewhere with modifications.12 Briefly, MTT was dissolved 0.5 mg/mL in DMEM without phenol red (PAA). Cells were washed three times with PBS and incubated with MTT at 37 °C for 2 h. MTT was discarded and dimethyl sulfoxide was added to the cells. The tissue plates were shaken at 200 rpm for 5 min. Adsorption was measured at 555 nm with Elx800 (BioTek, Bad Friedrichshall, Germany).
Trypan blue exclusion assay
After stimulation with aflibercept for 24 h or 7 days, cell viability was determined by trypan blue exclusion assay as previously described.13 Briefly, cells were detached with trypsin-EDTA, the pellet was resuspended in PBS and the number of viable cells were assessed in a Neubauer's cell chamber, using trypan blue as non-viability stain. The result was related to the number of cells of an untreated control which was considered 100%.
Scratch-assay
The scratch-assay determines the migratory ability of the cells. Primary RPE cells were seeded in a 12-well plate. Three wounds were scratched in the confluent cell layer with a toothpick and the cells were washed with PBS to remove detached cells. Medium without phenol red (Dulbecco's modified Eagle's medium) supplemented with penicillin/streptomycin (1%), HEPES (25 mM), sodium-pyruvate (110 mg/mL), and 10% fetal calf serum) was added and microscopic bright field pictures of three precise spots were taken and the coordinates noted (Zeiss, Jena, Germany). Aflibercept, ranibizumab, or bevacizumab, respectively, was added to the wells at indicated concentrations. Twenty-four hours after stimulation, another picture was taken at the same coordinates. To analyse the migration of the cells, the stimulation was conducted in duplicate and three pictures per well were made. The gap size of the wound was measured with AxioVision Rel.4.8. (Zeiss, Jena, Germany), and the percentages of coverage of the wounds were evaluated. Complete coverage was defined as 100%.
Immunocytochemistry
Immunocytochemistry of RPE cells was conducted as previously described with modifications.14 Briefly, cells were treated with 125 µg/mL or 500 µg/mL aflibercept. After the indicated time periods, cells were briefly washed with PBS 0.1% azide, and fixed in 2.5% paraformaldehyde at room temperature followed by acetone-ethanol for 10 min at −20°. Cells were blocked with 0.1% BSA 0.2% glycine in TBS, washed and incubated with goat anti-human antibodies coupled with AlexaFluor 555 (Invitrogen, Karlsruhe, Germany) at 37° for 1 h. Nuclear staining was performed using Hoechst stain. Cells were washed and mounted using Slow Fade Mounting Medium (Invitrogen). Stained cells were analysed with the Axio Imager Z1 (Zeiss, Jena, Germany).
Phagocytosis assay
Phagocytosis was investigated in an assay using FITC-labelled beads, treated with a crude extract of photoreceptor outer segments. The uptake was assessed in a fluorescence microscope, as previously described.14 Briefly, photoreceptor outer segments were prepared from porcine retina and used to opsonise FITC-labelled latex beads (diameter 1 µm) for 1 h at room temperature. Seven days after a single treatment with aflibercept (500 µg/mL) or bevacizumab (250 µg/mL), RPE cells of 2nd passage were treated with opsonised beads and incubated for 4 h at 37 °C. In this time frame, opsonised beads are readily taken up by RPE cells.14 Cellular uptake of beads was detected in fluorescence microscopy. For this, cells were fixed and prepared for fluorescence microscopy as described above, and eight pictures per slide were taken. Beads and nuclei of cells displaying intracellular aflibercept or bevacicumab, respectively, were counted, the ratio determined, and compared with untreated control.
Statistics
Each experiment was independently repeated at least three times. Graphs display mean and SD. Significance was evaluated with an unpaired, two-tailed Student t test. A p value of ≤0.05 was considered significant.
Results
Toxicity of aflibercept
Application of aflibercept at a concentration of 500 µg/mL for 24 h and 7 days, respectively, displayed no toxicity on RPE cells, neither in MTT-assay (24 h: 97.8±3.0%; 7 days: 102.4±0.9%) nor in trypan blue exclusion assay (24 h: 97.9±7.3%; 7 days: 97.2±17.4%) (figure 1).
Toxicity of 500 µg/mL aflibercept after 24 h and 7 days. Confluent primary porcine RPE cells of second or third passage were treated with aflibercept at a concentration of 500 µg/mL for 24 h and 7 days, respectively. Toxicity was evaluated in trypan blue exclusion assay and methyl thiazolyl tetrazolium (MTT) assay (n=6 for every assay). Aflibercept displayed no toxicity on RPE cells, neither in trypan blue-exclusion assay (A) nor in MTT assay (B). Significance was determined by Student t test. h=hours, d=days, afli=aflibercept, MTT=methyl thiazolyl tetrazolium.
Wound healing
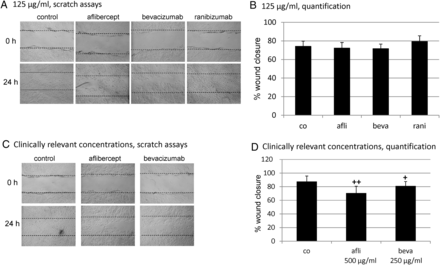
In the wound healing assay, we compared aflibercept, bevacizumab and ranibizumab at a concentration of 125 µg/mL. Additionally, we assessed aflibercept and bevacizumab at their clinically relevant concentrations (500 µg/mL and 250 µg/mL, respectively). At a concentration of 125 µg/mL, none of the tested reagents displayed a statistically significant effect on wound healing compared with control (control 74.2±5.5% closure, aflibercept: 72.2±6.1% closure; bevacizumab 71.6±5.0% closure; ranibizumab 79.3±6.3% closure). However, when clinically relevant concentrations were used, aflibercept and bevacizumab displayed a statistically significant effect on wound healing compared with control (control 87.3±8.4% closure, aflibercept 500 µg/mL 70.3±10.3% closure, p<0.01; bevacizumab 250 µg/mL 80.7±6.8% closure, p<0.05) (figure 2).
Wound healing assay. Migration ability was determined in a scratch assay where three wounds were scratched in the confluent RPE cell layer with a toothpick, microscopic bright field pictures were taken and the precise coordinates were reassessed after 24 h of stimulation with aflibercept, bevacizumab, or ranibizumab at indicated concentrations. Aflibercept, bevacizumab and ranibizumab at 125 µg/mL, (A) exemplary scratch and (B) quantification (n=5); clinical relevant concentrations of aflibercept (500 µg/mL) and bevacizumab (250 µg/mL), (C) exemplary scratch and (D) quantification (n=4). At a concentration of 125 µg/mL, none of the tested reagents displayed a statistically significant effect on wound healing compared with control. At clinically relevant concentrations, aflibercept and bevacizumab displayed a statistically significant effect on wound healing compared with control. Significance was determined by Student t test. ‘+’ p<0.05; ‘++’ p<0.01. co=control, afli=aflibercept, beva=bevacizumab, rani=ranibizumab.
Cellular uptake
Aflibercept was found in RPE cells after 1 h of stimulation and was intracellularly seen for at least 7 days (latest time point tested). After 1 h of incubation, a vesicle-like distribution was seen, with some but not all cells affected. After 3 days of incubation, a netlike pattern was observed, but vesicles were still present. No difference in pattern or intensity was found for the application of 125 µg/mL or 500 µg/mL aflibercept (figure 3). Additionally, no statistical significant differences were found between the percentage of cells stained positive for aflibercept at 125 µg/mL and 500 µg/mL (cells stained positive for aflibercept: 1 h: 125 µg/mL 82.9%±15.2%, 500 µg/mL 84.9%±8.9%; 4 h: 125 µg/mL 81.9%±15.1%, 500 µg/mL 84.9%±9.0%; 3 d: 125 µg/mL 83.2%±15%, 500 µg/mL 84.6±13.7%; 5 d: 125 µg/mL 86.6%±6.0%, 500 µg/mL 84.4%±7.4%; 7 d: 125 µg/mL 82.5%±14.3%, 500 µg/mL 88.2%±5.6%).
Intracellular uptake of aflibercept in RPE cells. RPE cells of second passage were treated with 125 µg/mL and 500 µg/mL, respectively, for various time periods (1 h, 4 h, 3 days, 5 days, 7 days). Intracellular aflibercept was detected in immunocytochemistry, using a goat anti-human antibody coupled with AlexaFluor555. Aflibercept can be found in RPE cells 1 h after stimulation and is still seen after 7 days, the latest investigated time point. Aflibercept is found in vesicles in the cells. Additionally, after 3 days, a netlike structure can be seen. No differences in cellular pattern or percentage of positive cells can be observed between 125 µg/mL and 500 µg/mL aflibercept (quantification done with n=6 independent experiments and 5 slides/experiment). Significance was determined by student's t test. h=hour/s, d=days.
Phagocytosis
We have previously shown that intracellular bevacizumab reduces the phagocytic capacity of RPE cells.9 In this study, cells positive for intracellular aflibercept displayed a significantly diminished phagocytosis of opsonised latex beads compared to untreated control (control: 42.8±4.3 beads/cell; aflibercept: 28.3±2.9; p<0.001). No differences between aflibercept-treated cells and bevacizumab-treated cells (30.2±5.2 beads/cell) were found (figure 4).
Influence of aflibercept (500 µg/mL) on the phagocytosis of RPE cells. RPE cells of second passage were treated once with 500 µg/mL aflibercept or 250 µg/mL bevacizumab. Phagocytosis was evaluated after 7 days in a phagocytosis assay utilising FITC-labelled beads, treated with a crude extract of photoreceptor outer segments, applied for 4 h to the cells. The uptake was assessed in a fluorescence microscope. Beads and nuclei of cells displaying intracellular aflibercept, or bevacicumab, respectively, were counted, the ratio determined and compared to untreated control (8 pictures per slide). Cells positive for intracellular aflibercept displayed a significantly diminished phagocytosis of opsonised latex beads compared to untreated control. No differences between aflibercept-treated cells and bevacizumab-treated cells could be determined. Significance was determined by Student t test. ‘+’ p<0.05, ‘+++’ p<0.001.
Discussion
We investigated the effect of aflibercept on the RPE physiology, focusing on toxicity, wound healing, intracellular uptake and phagocytosis.
In a recently published paper,15 the toxicity of aflibercept on several ocular cells, including the RPE cell line Arpe19, was tested. No toxicity was found in MTT assay or calcein stain up to a concentration of 1 mg/mL for 24 h. In another study, the toxicity of aflibercept up to a concentration of 2 mg/mL up to 72 h was investigated, using MTT assay, crystal violet staining and caspase 3/7 activation. No toxicity was seen. Our data correspond to these findings, as no toxic effect of aflibercept at a concentration of 500 µg/mL after 24 h or 7 days was found on primary porcine RPE cells in an MTT assay or a trypan blue exclusion assay. However, we did find a concentration-dependent effect on wound healing, as seen in a scratch assay of confluent primary RPE cells, which was seen at a concentration of 500 µg/mL, but not 125 µg/mL aflibercept. As we tested aflibercept, bevacizumab and ranibizumab at 125 µg/mL and at their clinically relevant concentrations, we found that bevacizumab displayed a similar concentration dependent effect seen at 250 µg/mL but not at 125 µg/mL. The clinically relevant dose of ranibizumab (125 µg/mL) did not exhibit any effect on wound healing. As all tested concentrations should be sufficient to completely block VEGF in the RPE cell culture supernatant,11 other possible effects of high concentrations of aflibercept or bevacizumab may be responsible, and it may be connected to the intracellular uptake of these substances (see below). The impairment of wound healing by aflibercept may indeed constitute an adverse effect of this drug which may be of clinical consequences. Peripheral RPE cells are able to migrate and divide in order to replace deceased RPE cells in the retina.16 Moreover, laser therapy induces wound-healing reactions in RPE cells which is considered to be part of the therapeutic effect of laser.17 ,18 This may be impaired by high concentrations of aflibercept or bevacizumab. Whether our in vitro findings are of clinical relevance in vivo, however, remains to be seen, especially as the clinically relevant concentrations refers to the intravitreal concentration immediately after injection, and may not reflect the actual concentration reached at the RPE.
We previously have shown that bevacizumab, but not ranibizumab, is taken up and stored by RPE cells.8 Recently, this has been confirmed for endothelial cells.10 In our study, an intracellular uptake of aflibercept in primary RPE cells can clearly be observed. Not all RPE cells take up aflibercept, which corresponds to the results obtained for bevacizumab. Intracellular aflibercept can be seen for up to 7 days (the last investigated time point), which also corresponds to bevacizumab.8 Aflibercept is seen in vesicles and, after 3 days, also in a net-like structure. The similarities between aflibercept and bevacizumab indicate that the Fc-part of the molecule may be involved in uptake and intracellular trafficking of the molecule. Interestingly, no differences were found between 125 µg/mL and 500 µg/mL aflibercept, indicating that the process is saturable, and that saturation is achieved. Of note, cellular uptake of aflibercept into the RPE cells has very recently been shown in vivo in monkeys.19
The uptake of aflibercept has consequences for the physiology of the cells as we see a significant reduction of phagocytosis in cells that incorporated aflibercept. Again, this mirrors the findings observed with bevacizumab and is clearly dependent on the presence of intracellular aflibercept and not on the inhibition of VEGF, as ranibizumab displays no effect on phagocytosis.9 As phagocytosis is an important task for the RPE cells in order to remove shed photoreceptor outer segments,20 a long-term inhibition of phagocytosis could, in fact, have a deleterious effect on photoreceptor function, as seen in RCS rats and other conditions where RPE phagocytosis is impaired.21 ,22 Subtle effects on a cellular level that cumulate in the long-term treatment may not be obvious in the short run, but could cause atrophic changes that would be indistinguishable for the natural course of the disease. However, the relevance of these findings for the in vivo situation remains to be seen, especially as enhanced atrophy could be found after 2 years of anti-VEGF treatment for bevacizumab and ranibizumab, which may even be more pronounced after ranibizumab treatment.23 ,24 In any case, anti-VEGF therapy should be closely monitored and an over-treatment should be avoided.
Signal transduction pathways altered by aflibercept which may convey its effects on RPE cells are not known. A connection may be found in ERK signalling, as ERK signalling is involved in migration and phagocytosis, and aflibercept has been shown to reduce ERK activation in endothelial cells, but the available data are insufficient to draw any conclusion on this matter, and further research needs to be conducted.25–27
In conclusion, aflibercept interferes with the physiology of RPE cells, as it impairs wound healing and is taken up into RPE cells, which is accompanied by a reduction of the phagocytic ability, indicating possible adverse effects of aflibercept.
References
Footnotes
-
Acknowledgements Parts of the data presented here have been presented at the DOG meeting 2013 and the WOC meeting 2014.
-
Contributors All authors meet the criteria for authorship. No other contributors are listed.
-
Funding This research was financially supported by a Novartis research grant.
-
Competing interests This research was financially supported by a Novartis research grant. AK has been a consultant for and received lecture fees and travel grants by Novartis Pharma.
-
Provenance and peer review Not commissioned; externally peer reviewed.
Linked Articles
- At a glance