Article Text
Abstract
Human serum-derived and plasma-derived therapies have become increasingly popular in the treatment of ocular surface disorders, with mounting clinical and scientific evidence suggesting good safety and efficacy profiles. These therapies may be considered for various ocular surface conditions, such as dry eye syndrome and persistent epithelial defect, when conservative management does not suffice. The costly and inconvenient process of obtaining the blood-derived products is the barrier to their more widespread use. Some blood-derived therapies, such as umbilical cord serum-derived and platelet-derived plasma preparations, may be more viable options since these therapies can be made readily available to patients. In this review, the existing literature on the safety and efficacy of blood-derived products, such as autologous serum tears, in the treatment of ocular surface diseases is discussed. Issues relevant to the production of autologous serum tears are also described.
- Cornea
- Ocular surface
- Tears
Statistics from Altmetric.com
Introduction
The health of the ocular surface depends on adequate maintenance of the tear film. The tear film's mucoaqueous and lipid layers provide mechanical protection of the corneal epithelium, while a variety of growth factors, vitamins, electrolytes and neuropeptides support the growth and migration of epithelial cells.1 ,2 Tear film abnormalities may occur in states of mucoaqueous deficiency, such as Sjögren's syndrome (SS) and goblet cell destruction, or with ocular adnexal problems, including meibomian gland deficiency and lagophthalmos. In ocular surface disorders, the mechanical and epitheliotrophic characteristics of the tear film are both crucial in preventing further compromise of epithelial integrity and in promoting wound healing.
Traditional, first-line methods of ocular surface disease treatment focus on shielding the epithelium from biomechanical trauma.2 Blood-derived therapies additionally contain some of the same growth factors, cytokines, vitamins and nutrients found in natural tears to support epithelial cell homeostasis, growth and migration.3 The use of blood-derived therapy for ocular surface disorders was first described in 1975 in a case series by Ralph and colleagues, in which a mobile ocular perfusion pump delivered serum or plasma to the ocular surfaces of six patients.4 Fox and associates5 then demonstrated the efficacy of autologous serum eye drops (ASE) in the treatment of keratoconjunctivitis sicca. This therapy gained more widespread attention after Tsubota and colleagues published two studies in 1999 showing convincing evidence of the benefits of ASE in SS-related dry eye syndrome (DES) and persistent epithelial defects (PEDs).6 ,7 Their studies were the first to systematically demonstrate both the efficacy and the safety of ASE.
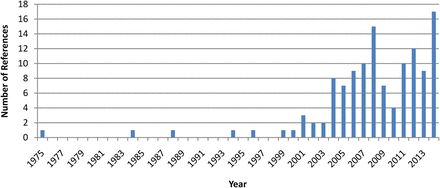
Since then, clinical research evaluating its effectiveness has expanded greatly8 (figure 1). Indications for which blood-derived therapy has been studied include DES caused by various aetiologies,6 ,9–27 PEDs,7 ,14 ,20 ,28–34 recurrent erosion syndrome,35 ,36 chemical injury37 ,38 and superior limbic keratoconjunctivitis.39 ASE, allogeneic serum, umbilical cord serum (UCS) and platelet-rich plasma (PRP) have all been studied for use in ocular surface disease. Barriers to their more common use include the potential for sample contamination, inconvenience to the patient of sample preparation and the lack of a standardised protocol for preparation.8 This review discusses the literature on human blood-derived treatments for ocular surface disease and surveys current methods for the production of ASE.
The number of peer-reviewed publications related to the use of human blood-related products in the treatment of ocular surface disease from October 1975 to December 2014. The search was conducted using PubMed with the key phrases ‘autologous serum tears’, ‘serum eye drops’, ‘serum-derived ocular’, ‘serum-derived eye’, ‘platelet-rich plasma eye’, ‘plasma rich in growth factors eye’, ‘cord serum eye’.
Autologous serum eye drops
ASE's biochemical properties are similar to those of human tears.1 ,40 Epidermal growth factor (EGF), which increases migration and proliferation of corneal epithelial cells, and transforming growth factor-β (TGF-β), which decreases epithelial cell proliferation, are the most important components of tears.3 ,41–43 Fibronectin, vitamin A and a variety of chemokines, growth factors and nutrients further contribute to the tear film milieu that maintains the ocular surface.3 Fibronectin is present at a concentration of 205 μg/mL in serum compared with a tear film concentration of 21 ng/mL.1 EGF concentrations are similar in serum (0.7–10 ng/mL) and tears (0.5 ng/mL),7 while vitamin A concentrations are much higher in serum (46 mg/mL) than in tears (0.02 mg/mL).1 More importantly, TGF-β concentrations are five times higher in serum than in tears.6 Therefore, many ophthalmologists prefer to use a 20% dilution of ASE to more closely match the TGF-β concentration in natural tears in order to prevent problems with epithelial cell proliferation.3 ,40 ,44
Dry eye syndrome
We found six prospective randomised controlled trials (RCTs) in the literature pertaining to ASE in the treatment of DES (listed in table 1).
Characteristics and outcomes of controlled clinical trials on autologous serum eye drop therapy in dry eye syndrome (DES)
The subjects and observers in RCTs on ASE therapy can be difficult to mask, because the placebo, usually artificial tears, is of a different colour than serum, and the viscosity of artificial tears may change after undergoing the freeze–thaw process.46 There also tends to be a strong placebo effect, since the artificial tears used in the control groups themselves have alleviating properties.
This was shown in the first controlled study by Tananuvat et al9 in 2001, in which 12 patients with bilateral severe dry eye of various aetiologies received artificial tears in one eye and ASE in the other. Although objective and subjective improvements were noted in the treatment eyes at 2 months, control eyes similarly improved, thus negating any differences between artificial tears and serum therapy. The study reported decreased use of concurrent topical lubricants from 11.5 drops per day to 2.15 drops per day, but this trend may have simply represented the necessity of decreasing eye drop frequency when applying ASE six times per day. Noble and colleagues,10 in contrast, found significant objective and subjective improvements in DES in their randomised controlled crossover trial including 16 patients with severe dry eye. Conjunctival impression cytology (CIC), performed by a masked pathologist, improved significantly on serum therapy. Dry eye symptoms were found to be managed significantly better after 3 months of serum therapy compared with after 3 months of artificial tears. However, the patients and observers were not masked to the treatments. Kojima and associates11 evaluated a shorter treatment length in a study of 20 dry eye patients with SS and without SS. They found that even with a 2-week treatment course, there was a significant improvement in subjective scores as well as tear break-up time (TBUT), rose-bengal staining scores and fluorescein staining scores in the treatment group. A potential shortcoming of this study was that although the observers were masked to which treatment the patients were receiving, the patients were aware of their own treatment group, which could have decreased drop compliance in the control group.
The two most recent RCTs by Urzua and colleagues12 and Celebi and associates13 employed the Ocular Surface Disease Index (OSDI) to better standardise their results. The OSDI has been validated as a reliable instrument for use in clinical trials.47 In the double-blind crossover study by Urzua and colleagues, 12 patients with severe DES were treated with 2 weeks of ASE and found to have no significant improvements in objective measures, including TBUT and OXFORD fluorescein staining score of the cornea. However, OSDI scores were improved after serum therapy by 50.95%, which was significantly better than the improvement on conventional treatment (22.19%). Celebi and colleagues13 corroborated the improvement in OSDI, as in their RCT of 20 patients with severe dry eye, OSDI scores decreased by 55.18% in the treatment group, compared with a decrease of 19.50% in the preservative-free artificial tears group.
Overall, the RCTs evaluating ASE for use in DES provide promising data for patients in terms of symptomatic relief of dry eye. More rigorous efforts to blind the subject and observer may decrease the potential for bias in future studies. Sample sizes of the current RCTs in the ASE literature were also too small to make definitive judgements on the efficacy of treatment.
Persistent epithelial defect
PED was among the first applications of ASE therapy to be studied7 and subsequent papers have demonstrated its efficacy in clinical practice.14 ,28–32 Poon and colleagues14 reported that out of 15 eyes with PEDs which had previously been treated for a mean length of time of 48.2 days aggressively with botulinum toxin-induced ptosis, bandage contact lenses, tarsorrhaphy and/or amniotic membrane transplantation, nine (60%) healed at a mean of 29 days after initiation of ASE. In another case series of 10 patients with PEDs (mean duration 22.4 days) caused by a variety of aetiologies, 6 (60%) PEDs healed within 1 week of ASE, while 2 (20%) did not heal by 1 month and 2 patients (20%) were lost to follow-up. Jeng and Dupps31 published on 25 eyes with PEDs of various aetiologies with median duration of 13.9 weeks, of which 23 (92%) healed within an average of 22.4 days. According to Tsubota and colleagues’7 grading system, treatment was effective (PED healed within 2 weeks) in 52% and partially effective (PED healed within 4 weeks) in 68% of cases. In this study, the longer the duration of the PED, the longer it took to heal with ASE therapy. Treatment of PED with a combination of ASE and silicone hydrogel contact lenses has also been explored.29 ,32 Schrader and collagues29 found that of six eyes with PEDs previously unsuccessfully treated, five (83.3%) healed within 1 month. Of note, an amorphous coating of nodular deposits was noted in three of these eyes and thought due to albumin protein deposition, which comprises 60% of serum protein content. No signs of infection were noted.
Based on the current literature, ASE therapy seems to be quite effective in the treatment of PEDs, but study sample sizes have been very small. As ASE preparations usually do not contain preservative, one must pay heed to the theoretically increased infection risk that could arise with a serum-derived product in the setting of an epithelial defect.
ASE production issues
Protocols
The process of ASE production varies and involves the collection of whole blood from patients, clotting, centrifugation, collection of the supernatant and dilution of the supernatant. According to a questionnaire about ASE production methods completed by 13 hospitals in Korea, amount of blood collected, clotting time, centrifugation speed and time, as well as per cent dilution and diluents differed between the hospitals.48 The NHS Blood and Transplant of Great Britain has employed a standardised method for production of ASE which has been used since 1997.49 However, its estimated yearly production cost per patient is US$4000 per year if a bottle is used and discarded each day.44
Few studies have directly compared clinical outcomes of different concentrations of ASE.21 ,43 In Cho and colleagues’ prospective study including 141 eyes with a DES or PED, PEDs healed significantly faster with 100% than with 50% ASE.23 Symptoms and corneal staining, but not TBUT or Schirmer test value, were also better with 100% than with 50% ASE at 12 weeks post-treatment in both the Sjögren’s and non-Sjögren’s groups. Liu and colleagues determined that a long clotting time (>120 min), centrifugation at 3000g for 15 min and dilution with balanced salt solution at 12.5–25% best supported cell proliferation, migration and differentiation of corneal epithelial cells.50 Based on this work, Partal and Scott44 proposed a low-cost protocol that would adhere to the US Food and Drug Administration requirements for ophthalmic use of autologous blood products. The estimated cost to supply 3 months of ASE to a patient in a safe manner, excluding costs of microbial testing, was US$195.19. Given the relative affordability and scientific plausibility of this protocol, further studies on ASE should use this protocol to determine its clinical performance.
As of now, ASE therapy is not widely accepted as the standard of care in most European countries and the USA, and since insurance plans will rarely cover its costs, patients must pay anywhere between US$25 and US$600 out of pocket for a 2–3 month supply. The Northern California Kaiser Permanente Health Care System, which covers a population of 3.2 million people, recently began to offer ASE as an insurance-covered benefit. Over a 1-year period, 103 patients were treated with 100% ASE, and of 30 patients with follow-up within 90 days, 27 (90%) reported an improvement of symptoms.20 Only two (6.7%) patients had transient discomfort with ASE.
Allogeneic preparations of serum eye drops
Autologous preparation of serum eye drops is inconvenient for the patient, and certain patients may not qualify for ASE treatment because of coexisting medical conditions, such as anaemia.25 An allogeneic sample of serum eye drops, on the other hand, can be prepared from already-stored whole blood in approximately 30 min.51 Table 2 shows data with regard to the use of allogeneic serum eye drops.
Characteristics and outcomes of clinical reports on allogeneic serum eye drop therapy
ABO antigens are known to exist on conjunctival and corneal surfaces, which may theoretically lead to an inflammatory reaction due to anti-A and anti-B antibodies in allogeneic serum.52 However, three of the four studies on allogeneic serum eye drops did not consider ABO blood type matching in their methods, and no adverse reactions were noted to occur. In New Zealand, where 39% of serum eye drops used since 2007 have been allogeneic, blood type AB donors whose serum therefore lacked anti-A and anti-B antibodies have been used in 73 patients with no reported adverse reactions.53 Given the potential of allogeneic serum to be as effective as autologous serum in the treatment of DES and PEDs, and its demonstrated safety without the need for matching donors with recipients, allogeneic serum may be a viable alternative to autologous serum in ocular surface disease management if more rigorously studied.
Complications
Preservatives are typically not added to ASE preparations because they can potentially cause epithelial toxicity. The high protein content of ASE may also predispose samples to microbial contamination.54 Clinical infection of the ocular surface by ASE was reported by Rocha and colleagues16 and Poon and colleagues14 in 2000 and 2001, respectively. Since then, there have been no known reported cases of ocular surface infections attributed to ASE. Reported rates of ASE sample contamination vary widely from 0% to 55%, and there was no trend towards higher rates of contamination at higher ASE concentrations.54–59 Lopez-Garcia and Garcia-Lozano found that adding sterilising filters to ASE containers decreased the contamination rate from 29% to 2%, thus decreasing the chances of clinical infection even further.59 Based on studies conducted by Fischer and colleagues58 and Bradley and colleagues60 to assess the biological stability of growth factors in ASE, it is recommended that samples be stored at −20°C for up to 6 months, and that single daily-use dropper containers be stored at 4°C between doses. Although clinical infection from ASE use is rare, possibly owing to immunologically active components within the serum, patients on ASE therapy, especially those simultaneously on topical steroids, should be closely monitored for signs of infection.56
Other complications from ASE use are similarly rare. McDonnell and associates described a patient who developed immunoglobulin deposition in the corneal stroma after application of ASE for 1 week for PED.61 Limbitis secondary to ASE use was reported by Welder and colleagues62 in a patient treated with ASE for atopic keratoconjunctivitis, which quickly resolved after the discontinuation of treatment.
Umbilical cord serum
UCS contains many of the same growth factors and components as natural tears, including EGF and vitamin A.33 UCS may serve as a viable alternative in patients who are not good candidates for ASE therapy, such as those with graft-versus-host disease (GVHD) or SS, where pro-inflammatory cytokines could be present in the serum.63 UCS has potential applications in the treatment of PEDs,33 GVHD-associated dry eye,18 SS-type DES,19 recurrent corneal erosions35 and chemical burns.37 ,38 Yoon and colleagues demonstrated that UCS eye drops were even more effective than ASE in terms of symptom and keratoepitheliopathy scores in a group of 92 eyes with severe DES at 1 and 2 months post-treatment initiation.19 Recurrent corneal erosions were also more effectively managed with UCS eye drops than with artificial tears alone in another study by Yoon and colleagues.35 A comparison study of UCS versus ASE and artificial tears in the treatment of ocular chemical burns found UCS-treated patients to require significantly less days to complete corneal epithelialisation than those treated with ASE or artificial tears.38 Since one sample of UCS may be distributed to many patients, preparation of samples can be done in advance and made readily available to patients. However, UCS samples must be rigorously screened for bloodborne infections prior to donation.
Platelet-derived plasma preparations
Platelet-derived plasma preparations have been used successfully in oral and maxillofacial surgery and orthopaedics to aid in wound healing, because of the high levels of growth factors found in platelets.64–66 Preparation methods of these solutions for ocular use vary in the literature. Whole blood samples are typically obtained, an anticoagulant is added and platelet membranes are disturbed by centrifugation with subsequent retrieval of a supernatant (plasma), which contains the growth factors.34 ,67–75 ‘Platelet releasate’ contains growth factors released from platelets by thrombin.68 ‘Plasma rich in growth factors’ (PRGF) does not include thrombin.69 ,71 ‘Platelet-rich plasma’ is produced with an additional centrifugation step.70
Promising in vitro and animal model data on platelet-derived plasma preparations led to the initiation of clinical studies evaluating their effects on PEDs and DES.34 ,68–71 ,74 ,75 Lopez-Plandolit and associates saw a PED healing rate of 85% of patients (17 of 20 eyes) in an average of 10.9 weeks on treatment with PRGF.75 Of note, six of the patients in their study were previously unsuccessfully treated with ASE. However, additional treatments were continued in most patients, two patients required lateral tarsorrhaphy and one required an amniotic membrane graft, thus making it somewhat difficult to assess whether there was an isolated effect of PRGF. Importantly, this study demonstrated the tolerability of PRGF, as only one patient had ocular discomfort requiring discontinuation. Platelet-derived plasma therapy's efficacy in PEDs was also compared with that of ASE in a clinical study of 28 eyes with PEDs from infectious keratitis.34 All 11 eyes treated with PRP achieved complete re-epithelialisation, whereas only 12 of 17 achieved the same in the ASE group. Healing rates were also faster in the PRP group, a difference which nearly reached statistical significance (p=0.059). Lopez-Plandolit's group also studied 16 patients with moderate to severe DES who received PRGF treatment for at least 3 months.74 Symptomatic improvement was noted, with a significant decrease in Dry Eye Questionnaire score after treatment, but objective findings, including lissamine green dye staining and CIC, were not affected by treatment.
Although evaluation of platelet-derived plasma therapies for ocular surface disease is currently in its early stages, these therapies have a good safety profile and may have superior efficacy when compared with ASE in the treatment of PEDs.
Conclusions
The use of human blood-derived therapies for treatment of ocular surface disorders has become more popular with clinical and scientific evidence that suggests good safety and efficacy profiles. Early application of such therapy may be warranted in certain situations. Barriers to their more widespread use include cost and the inconvenient process of obtaining the products. Some blood-derived therapies, such as UCS and platelet-derived plasma preparations, may be more viable options since these therapies can be made readily available to patients. Further research using standardised protocols for the production of blood-derived treatments will provide more definitive data on their efficacy, and may ultimately lead to more widespread acceptance of these therapies in everyday practice.
References
Footnotes
Contributors BHJ was commissioned to write this review. The initial draft was prepared by NGS under the direct supervision of BHJ, and critical revision was provided by BHJ over multiple revisions.
Competing interests None declared.
Provenance and peer review Commissioned; externally peer reviewed.