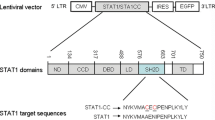
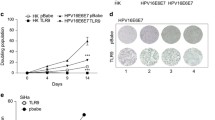
Summary
Recombinant human interferon-β ser17 (IFN-β ser17), a cytokine that exhibits both antiviral and antiproliferative activity against a wide variety of cell types, causes a time- and dose-dependent inhibition of monolayer growth and of the expression of the c-myc proto-oncogene in DLD-l Clone A human colon-carcinoma cells. The suppression of c-myc expression mediated by IFN-β ser17 is due to a posttranscriptional destabilization of c-myc mRNA rather than to an inhibition of c-myc mRNA transcription. There is evidence suggesting that the selective reduction in the half-life of c-myc mRNA in IFN-β ser17-treated cells occurs through an increase in the activity of the 2′,5′-oligoadenylate synthetase/RNase L [2′,5′-oligo (A) synthetase] pathway in DLD-1 Clone A cells. Cotreatment of these cells with IFN-β ser17 and the anticancer agentN-methylformamide leads to the partial abrogation of 2′,5′-oligo (A) synthetase activity and the stabilization of c-myc mRNA. These findings suggest that there is a correlation between the IFN-β ser17-mediated suppression of c-myc expression and the induction of 2′,5′-oligo (A) synthetase activity in DLD-1 clone A cells.
Similar content being viewed by others
References
Bishop JM (1987) The molecular genetics of cancer. Science 235: 305–312
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–251
Brown GE, Lebleu B, Kawakita M, Shamla S, Sen GC, Lengyel P (1976) Increased endonuclease activity in an extract from mouse Ehrlich ascites tumor cells that had been treated with a partially purified interferon preparation: dependence on double-stranded RNA. Biochem Biophys Res Commun 69:114–122
Chapekar MS, Glazer RI (1983) Effects of fibroblast and recombinant leukocyte interferons and double-stranded RNA on ppp(2′,5′)An synthesis and cell proliferation in human colon carcinoma cells in vitro. Cancer Res 43:2683–2687
Chapekar MS, Glazer RI (1984) Effects of human immune interferon on cell viability, (2′,5′)oligoadenylate synthesis, and polyamine-dependent protein phosphorylation in human colon carcinoma cells in vitro. Cancer Res 44:2144–2149
Chapekar MS, Glazer RI (1985) Synergistic effect of human immune interferon and double-stranded RNA against human colon carcinoma cells in vitro. Cancer Res 45:2539–2544
Chapekar MS, Glazer RI (1986) Potentiation of the cytocidal effect of human interferon by different synthetic double-stranded RNAs in the refractory human colon carcinoma cell line BE. Cancer Res 46: 1698–1702
Chatterjee D, Mendelsohn AM, Shank PR, Savarese TM (1989) Reversible suppression of c-myc expression in a human colon carcinoma cell line by the anticancer agentN-methylformamide. Cancer Res 49:3910–3916
Chirgwin JM, Prybyla AE, MacDonald RJ, Rutter WJ (1979) Isolation of a biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry 18: 5294–5299
Chousterman S, Chelbi-Alix MK, Thang MN (1987) 2′,5′-Oligoadenylate synthetase expression is induced in response to heat shock. J Biol Chem 262:4806–4811
Cordeiro RF, Savarese TM (1986) Role of glutathione depletion in the mechanism of action ofN-methylformamide andN,N-dimethylformamide in a cultured human colon carcinoma cell line. Cancer Res 46:1297–1305
Dani C, Blanchard JM, Piechaczyk M, El Sabouty S, Marty L, Jeanteur P (1984) Extreme instability ofmyc mRNA in normal and transformed human cells. Proc Natl Acad Sci USA 81:7046–7050
Dani C, Mechti N, Piechaczyk M, Lebleu B, Jeanteur P, Blanchard JM (1985) Increased rate of degradation of c-myc mRNA in interferon-treated Daudi cells. Proc Natl Acad Sci USA 82:4896–4899
DeBenedetti A, Pytel BA, Baglioni C (1987) Loss of (2′,5′)oligoadenylate synthesis activity by the production of antisense RNA results in lack of protection by interferon from viral infections. Proc Natl Acad Sci USA 84:658–662
Einat M, Resnitsky D, Kimchi A (1985) Close link between the reduction of c-myc expression by interferon and G0/G1 arrest. Nature 313:597–600
Erisman MD, Rothberg PH, Diehl RE, Morse CC, Spendorfer JM, Astrin SM (1985) Deregulation of c-myc gene expression in human colon carcinoma is not accompanied by amplification or rearrangement of the gene. Mol Cell Biol 5:1969–1976
Erisman MD, Scott JK, Watt RA, Astrin SB (1988) The c-myc protein is constitutively expressed at elevated levels in colorectal carcinoma cell lines. Oncogene 2:367–371
Erisman MD, Scott JK, Astrin SM (1989) Evidence that the familial adenomatous polyposis gene is involved in a subset of colon cancers with complementable defect in c-myc regulation. Proc Natl Acad Sci USA 86:4264–4268
Fidler IJ, Heicappell R, Saiki I, Grutter MG, Horisberg MA, Nuesch J (1987) Direct antiproliferative effects of recombinant interferon-alpha B/D hybrids on human tumor cell lines. Cancer Res 47: 2020–2027
Greenberg ME, Ziff EB (1984) Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature 311:433–438
Hamburger AW, Condon ME, O'Donnell K (1988) Inhibition of mitogen-stimulated growth of human colon cancer cells by interferon. Br J Cancer 58:147–151
Hayward WS, Neal B, Astrin SM (1981) Alteration of a cellularonc gene by promotor insertion in ALV-induced lymphoid leukosis. Nature 290:475–480
Jonak GJ, Knight E Jr (1984) Selective reduction of c-myc mRNA in Daudi cells by human beta-interferon. Proc Natl Acad Sci USA 81: 1747–1750
Kelly JM, Gilbert CS, Stark GR, Kerr IM (1985) Differential regulation of interferon-induced mRNAs and c-myc mRNA by α- and γ-interferons. Eur J Biochem 153:367–371
Kelly K, Cochran BH, Stiles CD, Leder P (1983) Cell specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell 35:603–610
Kimchi A (1987) Autocrine interferon and the suppression of the c-myc nuclear oncogene. In: Gresser I (ed) Interferon 8. Academic Press, New York, p 86
Knight E, Anton ED, Fahey D, Friedland BK, Jonak GJ (1985) Interferon regulates c-myc gene expression in Daudi cells at the post-transcriptional level. Proc Natl Acad Sci USA 82:1151–1154
Lengyel P (1982) Biochemistry of interferons and their actions. Annu Rev Biochem 51:251–282
Lilis PK, Brown TD, Beougher K, Koeller J, Marcus SG, Von Hoff DD (1987) Phase II trial of recombinant beta interferon in advanced colorectal cancer. Cancer Treat Rep 71:965–967
Linial M, Gunderson N, Groudine M (1985) Enhanced transcription of c-myc in bursal lymphoma cells requires continuous protein synthesis. Science 220:1126–1132
McMahon M, Stark GR, Kerr IM (1986) Interferon-induced gene expression in wild-type and interferon-resistant human lymphoblastoid (Daudi) cells. J Virol 57:362–366
Mehmet H, Taylor-Papadimitrou J, Rozengurt E (1989) Interferon inhibition of bombesin-stimulated mitogenesis in Swiss 3T3 cell access without blocking c-fos and c-myc expression. J Interferon Res 9:205–213
Merritt JA, Borden EC, Ball LA (1985) Measurement of 2′,5′-oligoadenylate synthetase in patients receiving interferon alpha. J Interferon Res 5:191–198
Morikawa K, Morikawa R, Killion JJ, Fan D, Fidler IJ (1990) Isolation of human colon carcinoma cells for resistance to a single interferon associated with cross-resistance to multiple recombinant interferons: alpha, beta, and gamma. J Natl Cancer Inst 82:517–522
Nepveau A, Marcu KB (1986) Intragenic pausing and antisense transcription within the murine c-myc locus. EMBO J 5:2859–2865
Papp KA, Floyd-Smith G (1989) Effects of interferon-β on Daudi cells and small-cell lung carcinoma cells which over-express the c-myc oncogene. Anticancer Res 9:1737–1742
Pestka S, Langer JA, Zoon KC, Samuel CE (1987) Interferons and their actions. Annu Rev Biochem 56:727–778
Pfizenmaier K, Bartsch H, Scheurich P, Seliger B, Ucer U, Vehmeyer K, Nagel GA (1985) Differential gamma-interferon response of human colon carcinoma cells: inhibition of proliferation and modulation of immunogenicity as independent effects of gamma interferon on tumor cell growth. Cancer Res 45:3502–3509
Pientenpol JA, Howe PH, Cunningham MR, Leof EB (1989) Interferon alpha/beta modulation of growth factor-stimulated mitogenicity in AKR-2B fibroblasts. J Cell Physiol 141:453–460
Resnitsky D, Yarden A, Zipori D, Kimchi A (1986) Autocrine beta-related interferon controls c-myc suppression and growth arrest during hematopoietic cell differentiation. Cell 46:31–40
Rigby PWH, Dieckman M, Rhodes C, Berg P (1979) Labelling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase. J Mol Biol 113:237–241
Saito H, Hayday AC, Wilman K, Hayward W, Tonegawa S (1983) Activation of the c-myc gene by translocation: a model for translational control. Proc Natl Acad Sci USA 80:7476–7480
Salzberg S, Hacohen D, David S, Dovrat S, Ahwan S, Gamliel H, Birnbaum M (1990) Involvement of the interferon-system in the regulation of cell growth and differentiation. Scan Microsc 4: 479–489
Samuel CE (1979) Mechanism of interferon action: phosphorylation of protein synthesis initiation factor eIF-2 in interferon-treated human cells by a ribosome-associated kinase processing site similar to hemin-regulated rabbit reticulocyte kinase. Proc Natl Acad Sci USA 76:600–604
Samuel CE (1987) Molecular mechanisms of interferon action. In: Stringfellow DA (ed) Clinical application of interferon and their inducers. Marcel Dekker, New York, p 1
Sarna G, Pertcheck M, Figlin R, Ardalan B (1986) Phase I study of recombinant β-ser 17 interferon in the treatment of cancer. Cancer Treat Rep 70:365–372
Schiller JH, Groveman DS, Schmid SM, Willson JKV, Cummings KB, Borden EC (1986) Synergistic antiproliferative effects of human recombinant alpha54- or beta-ser interferon with gamma-interferon on human cell lines with various histogenesis. Cancer Res 46: 483–488
Schiller JH, Bittner G, Storer B, Willson JKV (1987) Synergistic antitumor effects of tumor necrosis and gamma-interferon on human colon carcinoma cell lines. Cancer Res 47:2809–2813
Schiller JH, Storer B, Willson KVJ, Borden EC (1987) Phase I trial of combinations of recombinant interferons 20-1 and γ in patients with advanced malignancy. Cancer Treat Rep 71:945–952
Schillbach K, Pollwein P, Scwab M, Handgreitner R, Treuner J, Niethammer D, Bruchett G (1990) Reduction of c-myc expression by antisense RNA is amplified by interferon: possible involvement of the 2–5 A system. Biochem Biophys Res Commun 170:1242–1248
Simpson RU, Hsu T, Beley DA, Mitchell BS, Alizadeh BN (1987) Transcriptional regulation of the c-myc proto-oncogene by 1,25 dihydroxy-vitamin D3 in HL-60 promyelocytic leukemia cells. J Biol Chem 262:4104–4108
Tonnigawa S, Lengyel P (1985) β-Interferon alters the pattern of proteins secreted from quiescent and platelet-derived factor-treated Balb C/3T3 cells. J Biol Chem 260:1975–1978
Tsuboi K, Hirayoshi K, Takeuchi K, Salse H, Shimada Y, Oshio G, Tobe T, Hatanaka M (1987) Expression of the c-myc gene in human gastrointestinal malignancies. Biochem Biophys Res Commun 146: 699–704
Vandenbussche P, Divizia M, Verhaegen-Lewalle J, Fuse A, Kuwata T, DeClerq E, Content J (1981) Enzymatic activities induced by interferon in human fibroblast cell lines differing in their sensitivity to the anticellular activity of interferon. Virology 111:11–22
Vennstrom B, Sheiness D, Zabiolki J, Bishop JM (1982) Isolation and characterization of c-myc, a cellular homolog of the oncogene (v-myc) of avian myelocytomatosis virus strain 29. J Virol 42: 773–779
Verhaegen-Lewalle M, Kuwata T, Zhang ZX, DeClerq E, Cantell K, Content J (1982) 2–5A synthetase activity induced by interferon alpha, beta, gamma in human cell lines differing in their sensitivity to the anticellular and antiviral activities of these interferons. Virology 117:425–434
Zullo JN, Cochran BH, Huang AS, Stiles CD (1985) Platelet-derived growth factor and double-stranded ribonucleic acids stimulate expression of the same genes in 3T3 cells. Cell 43:793–800
Author information
Authors and Affiliations
Additional information
This investigation was supported by PHS grants CA 38630 and CA 20892, by Research Career Development Award CA 01241 awarded by the National Cancer Institute, DHHS, and by grant BC-640 from the American Cancer Society (to T.M.S.). Support was also provided by an Advanced Predoctoral Fellowship Award from the Pharmaceutical Manufacturers Association (to D.C.)
Rights and permissions
About this article
Cite this article
Chatterjee, D., Savarese, T.M. Posttranscriptional regulation of c-myc proto-oncogene expression and growth inhibition by recombinant human interferon-β ser17 in a human colon carcinoma cell line. Cancer Chemother. Pharmacol. 30, 12–20 (1992). https://doi.org/10.1007/BF00686479
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00686479