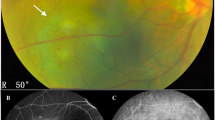
Abstract
Introduction: Histologically demonstrable microcirculation patterns (microcirculation pattern) of human choroidal melanomas have prognostic significance for the patient. We report on our experience in imaging these microcirculation pattern in vivo using simultaneous confocal Fluorescein (FA)- and Indocyaninegreen (ICG) angiography before and after brachytherapy.Patients and methods: The simultaneously procuredconfocal FA- and ICG angiograms of 50 patients with untreated choroidal melanomaswere studied for the visibility of microcirculation pattern. Patients were also followedwith simultaneous FA/ICG after brachytherapy.Results: Confocal FA disclosed signs of tumor vascularization in 12 (24%) of the 50 examined patients but microcirculation pattern only in 3 patients (6%). In contrast, simultaneously obtained confocal ICG disclosed microcirculation pattern in 47 patients (94%). In 10 (77%) of the 13 patients the tumor microcirculation changed considerably after brachytherapy: Distortion, thickening, thinning, as well as complete obliteration of vessels could be observed.Conclusion: Histologically demonstrated microcirculation pattern can be imaged in vivo. This offers the possibility to assess the likely biologic behavior of the individual tumor without the need for obtaining a cytologic or histologic specimen via enucleation or fine-needle biopsy. Confocal ICG angiogiography images microcirculation pattern better than FA which can be explained by the different absorption-, fluorescence- and exudation-characteristics ICG. Follow-up with confocal ICG of choroidal melanomas after brachytherapy shows different features and allows for visualization of themicrocirculation reaction to the treatment which might be a useful tool for studying the effects of future anti-angiogenesis based tumor therapies.
Similar content being viewed by others
References
Folberg R, Pe'er J, Gruman L, et al. The morphologic characteristics of tumor blood vessels as a marker of tumor progression in primary human uveal melanoma: a matched case-control study. Hum Pathol 1992; 23: 1298–1305.
Folberg R, Rummelt V, Parys, et al. The prognostic value of tumor blood vessel morphology in primary uveal melanoma. Ophthalmology 1993; 100: 1389–1398.
Folberg R, Mehaffey M, Gardner L, et al. Themicrocirculation of choroidal and ciliary body melanomas. Eye 1997; 11: 227–238.
Rummelt V, Folberg R, Rummelt C, et al. Microcirculation architecture of melanocytic nevi and malignant melanomas of the ciliary body and choroid. Ophthalmology 1994; 101: 718–727.
Rummelt V, Gardner L, Folberg R, et al. Three-dimensional relationships between tumor cells and microcirculation with double cyanine immunolabeling, laser scanning confocal microscopy, and computer-assisted reconstruction: an alternative to cast corrosion preparations. J Histochem Cytochem 1994; 42: 681–686.
Rummelt V, Folberg R, Woolson R, et al. Relation between the microcirculation architecture and the aggressive behavior of ciliary body melanomas. Ophthalmology 1995; 102: 844–851.
Char D, Kroll S, Crawford J, et al. Uveal melanoma cycling, vascular patterns and prognosis. Inv Ophth Vis Sci 1994; 35: 17–21.
Sakamoto T, Sakamoto M, Yoshikawa H, et al. Histologic findings and prognosis of uveal malignant melanoma in japanese patients. American Journal of Ophthalmology 1996; 121: 276–283.
Seregard S, Spanberg B, Juul C, Osskarsson M. Prognostic accuracy of the mean of the largest nucleoli, vascular patterns, and PC-10 in posterior uveal melanoma. Ophthalmology 1998; 105: 485-491.
Mueller AJ, Bartsch DU, Folberg R, et al. Imaging the microvasculature of choroidal melanomas with confocal indocyanine green scanning laser ophthalmoscopy. Arch Ophthalmol 1998; 116: 31–39.
Mueller AJ, Folberg R, W. F, et al. Evaluation of the human choroidal melanoma rabbit model for microvascularization patterns. Exp Eye Res 1999; 68: 671–678.
Mueller AJ, W. F, Folberg R, et al. Evaluation of microvascularization pattern visibility in human choroidal melanomas comparing simultaneously taken confocal Fluorescein with Indocyanine green angiograms. Graefe's Arch Clin Exp Ophthalmol 1999; 237: 448–456.
Edwards MG, Schachat AP. Tumors in the posterior pole. International Ophthalmology Clinics 1995; 35: 123–135.
Flindall R, Gass J. A histopathologic fluorescein angiographic correlative study of malignant melanomas of the choroid. Can J Ophthalmol 1971; 6: 258–267.
Lommatzsch P, Ballin R, Helm W. Fluorescein angiography in the follow-up study of choroidal melanoma after 106Ru/106Rh plaque therapy. Retina 1987; 7: 148–155.
Pettit T, Barton A, Foos R, Christensen R. Fluorescein angiography of choroidal melanomas. Arch Ophthalmol 1970; 83: 27–38.
Tarkkanen A, Laatikainen L. Fluorescein angiography in the long-term follow-up of choroidal melanoma after conservative treatment. Acta Ophthalmol 1985; 63: 73–79.
Flower R. Infrared absorption angiography of the choroid and some observations on the effects of high intraocular pressures. Am J Ophthalmol 1972; 74: 600–614.
Flower R, Hochheimer B. Clinical infrared absorption angiography of the choroid. Am J Ophthalmol 1972; 73: 458–459.
Flower R. Injection technique for indocyanine green and sodium fluorescein dye angiography of the eye. Invest Ophthalmol Vis Sci 1973; 12: 881–895.
Flower R, Hochheimer B. Indocyanine green dye fluorescence and infrared absorption choroidal angiography performed simultaneously with fluorescein angiography. Johns Hopkins Med J 1976; 138: 3–42.
Hochheimer B. Angiography of the retina with Indocyanine green. Arch Ophthalmol 1971; 86: 564–565.
Bischoff P, Flower R. Ten years experience with choroidal angiography using indocyanine green dye: a new routine examination or an epilogue? Doc Ophthalmol 1985; 60: 235–239.
Chopdar A, Turk A, Hill D. Fluorescent infra-red angiography of the fundus oculi using indocyanine green dye. Trans Ophthalmol Soc Uk 1978; 98: 142–146.
Bartsch DU, Weinreb R, Zinser G, Freeman W. Confocal scanning infrared laser ophthalmoscopy for indocyanine green angiography. Am J Ophthalmol 1995; 120: 642–651.
Freeman WR, Bartsch DU, Mueller AJ, et al. Simultaneous indocyanine green and fluorescein angiography using a confocal scanning laser ophthalmoscope. Archives of Ophthalmology 1998; 116: 455–463.
Albert D, Marcus D. Accuracy of diagnosis of choroidal melanomas in the collaborative ocular melanoma study. Arch Ophthalmol 1990; 108: 1268–1273.
Mitchell M, Harel W, JK-M, et al. Active specific immunotherapy of melanoma with allogeneic cell lysates. Rationale, results, and possible mechanisms of action. Ann NY Acad Sci 1993; 690: 153.
Quan W, Mitchell M. Phase II trial of carbetimer in metastatic melanoma. Invest New Drugs 1993; 11: 231–233.
Schaller U, Mueller AJ, Bosserhoff A, et al. Melanoma inhibiting activity (MIA): Evaluierung eines neuen tumorassozierten Antigens als Serummarker für uveale Melanome. Ophthalmologe, in press.
Crawford J, Char D. Histopathology of uveal melanomas treated with charged particle radiation. 1987; 94: 639–643.
MacFault P, Morgan G. Histopathological changes in malignant melanomas of the choroid after cobalt plaque therapy. Brit J Ophthalmol 1977;61:221-8.
Messmer E, Bornfeld N, Foerster M, et al.Histopathologic findings in eyes treated with a ruthenium plaque for uveal melanoma. Graefes Arch Clin Exp Ophthalmol 1992; 230: 391–396.
Shields C, Shields J, Karlsson U, et al. Enucleation after plaque radiotherapy for posterior. Ophthalmol 1990; 97: 1665–1670.
Seddon J, Gragoudas E, Albert D. Ciliary body and choroidal melanomas treated by proton beam irradiation. Arch Ophthalmol 1983; 101: 1402–1408.
Casey R, Li W. Factors controlling ocular angiogenesis. Am J Ophthalmol 1997; 124: 521–529.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mueller, A., Bartsch, DU., Schaller, U. et al. Imaging the microcirculation of untreated and treated human choroidal melanomas. Int Ophthalmol 23, 385–393 (2001). https://doi.org/10.1023/A:1014471118208
Issue Date:
DOI: https://doi.org/10.1023/A:1014471118208