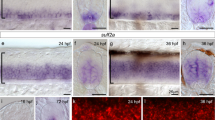
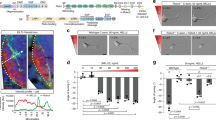
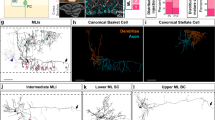
Abstract
Although cell migration is crucial for neural development, molecular mechanisms guiding neuronal migration have remained unclear. Here we report that the secreted protein Slit repels neuronal precursors migrating from the anterior subventricular zone in the telencephalon to the olfactory bulb. Our results provide a direct demonstration of a molecular cue whose concentration gradient guides the direction of migrating neurons. They also support a common guidance mechanism for axon projection and neuronal migration and suggest that Slit may provide a molecular tool with potential therapeutic applications in controlling and directing cell migration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hardesty, I. On the development and nature of the neuroglia. Am. J. Anat. 3, 229–268 (1904).
Cajal, S. R. Histology of the Nervous System (trans. Swanson, N. & Swanson, L. W.) (Oxford Univ. Press, New York, 1995).
Rakic, P. Neuron–glia relationship during granule cell migration in developing cerebellar cortex. J. Comp. Neurol. 141, 283–312 (1971).
Rakic, P. Guidance of neurons migrating to the fetal monkey neocortex. Brain Res. 33, 471–476 (1971).
Rakic, P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J. Comp. Neurol. 145, 61–84 (1972).
Tilney, F. Behavior in its relation to the development of the brain. Part II. Correlation between the development of the brain and behavior in the albino rat from embryonic states to maturity. Bull. Neurol. Inst. NY 3, 252–358 (1933).
Levi-Montalcini, R. The origin and development of the visceral system in the spinal cord of the chick embryo. J. Morphol. 86, 253–278 (1950).
Angevine, J. B. J & Sidman, R. L. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature 192, 766–768 (1961).
Hatten, M. E. & Liem, R. H. K. Astroglia provide a template for the positioning of developing cerebellar neurons in vitro. J. Cell Biol. 90, 622–630 (1981).
Hatten, M. E., Liem, R. H. K. & Mason, C. A. Two forms of glial cells interact differently with neurons in vitro. J. Cell Biol. 98, 193–204 (1984).
Gray, G. E., Leber, S. M. & Sanes, J. R. Migratory patterns of clonally related cells in the developing central nervous system. Experientia 46, 929–940 (1990).
Walsh, C. & Cepko, C. Cell lineage and cell migration in the developing cerebral cortex. Experientia 46, 940–947 (1990).
Norman, M. G., McGillivray, B. C., Kalousek, D. K., Hill, A. & Poskitt, K. J. in Congenital Malformations of the Brain: Pathologic, Embryological, Clinical, Radiological and Genetic Aspects (ed. Norman, M. G.) 223–277 (Oxford Univ. Press, New York, 1995).
Gordon, N. Epilepsy and disorders of neuronal migration. II: Epilepsy as a symptom of neuronal migration defects. Dev. Med. Child. Neurol. 38, 1131–1134 (1996).
Pearlman, A. L., Faust, P. L., Hatten, M. E. & Brunstrom, J. E. New directions for neuronal migration. Curr. Opin. Neurobiol. 8, 45–54 (1998).
Falconer, D. S. Two new mutants ‘trembler’ and ‘reeler’, with neurological actions in the house mouse. J. Genet. 50, 192–201 (1951).
D'Arcangelo, G.et al. Aprotein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature 374, 719–723 (1995).
Hirotsune, S.et al. The reeler gene encodes a protein with an EGF-like motif expressed by pioneer neurons. Nature Genet. 10, 77–83 (1995).
Luskin, M. B. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron 11, 173–189 (1993).
Lois, C. & Alvarez-Buylla, A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc. Natl Acad. Sci. USA 90, 2074–2077 (1993).
Frotscher, M. Cajal-Retzius cells, Reelin, and the formation of layers. Curr. Opin. Neurobiol. 8, 570–575 (1998).
Rio, C., Rieff, H. I., Qi, P. & Corfas, G. Neuregulin and erbB receptors play a critical role in neuronal migration. Neuron 19, 39–50 (1997).
Farbman, A. I. in Smell and Taste in Health and Disease (eds Getchell, T. V. et al.) 19–32 (Raven, New York, 1991).
Altman, J. & Das, G. D. Autoradiographic and histological studies of postnatal neurogenesis I: a longitudinal investigation of the kinetics, migration and transformation of cells incorporating tritiated thymidine in neonate rats, with special reference to postnatal neurogenesis in some brain regions. J. Comp. Neurol. 127, 337–390 (1966).
Hinds, J. W. Autoradiographic study of histogenesis in the mouse olfactory bulb. II cell proliferation and migration. J. Comp. Neurol. 134, 305–322 (1968).
Altman, J. Autoradiographic and histological studies of postnatal neurogenesis IV: cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J. Comp. Neurol. 137, 433–458 (1969).
Lois, C. & Alvarez-Buylla, A. Long-distance neuronal migration n the adult mammalian brain. Science 264, 1145–1148 (1994).
Lois, C., Garcia-Verdugo, J.-M. & Alvarez-Buylla, A. Chain migration of neuronal precursors. Science 271, 978–981 (1996).
Zigova, T.et al. Acomparison of the patterns of migration and the destinations of homotopically transplanted neonatal subventricular zone cells and heterotopically transplanted telencephalic ventricular zone cells. Dev. Biol. 173, 459–474 (1996).
Hu, H., Tomasiewics, H., Magnuson, T. & Rutishauser, U. The role of polysialic acid in migration of olfactory bulb interneurons precursors in the subventricular zone. Neuron 16, 735–743 (1996).
Hu, H. & Rutishauser, U. Aseptum-derived chemorepulsive factor for migrating olfactory interneuron precursors. Neuron 16, 933–940 (1996).
Wichterle, H., Garcia-Verdugo, J. M. & Alvarez-Buylla, A. Direct evidence for homotypic, glia-independent neuronal migration. Neuron 18, 779–791 (1997).
Kleinman, H. K., McGravey, M. L., Liotta, L. A., Robey, P. G., Tryggvason, K. & Martin, G. R. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry 21, 6188–6193 (1982).
Li, H. S.et al. Vertebrate Slit, a secreted ligand for the transmembrane protein roundabout, is a repellent for olfactory bulb axons. Cell 96, 807–818 (1999).
Nguyen Ba-Charvet, K. T.et al. Slit2-mediated chemorepulsion and collapse of developing forebrain axons. Neuron 22, 463–473 (1999).
Wang, K. H.et al. Purificaton of an axon elongation- and branch-promoting activity from brain identifies a mammalian Slit protein as a positive regulator of sensory axon growth. Cell 96, 771–784 (1999).
Kidd, T., Bland, K. S. & Goodman, C. S. Slit is the midline repellent for the Robo receptor in Drosophila. Cell 96, 785–794 (1999).
Brose, K.et al. Evolutionary conservation of the repulsive axon guidance function of Slit proteins and of their interactions with Robo receptors. Cell 96, 795–806 (1999).
Krull, C. E.et al. Interactions of Eph-related receptors and ligands confer rostrocaudal pattern to trunk neural crest migration. Curr. Biol. 7, 571–580 (1997).
Smith, A., Robinson, V., Patel, K. & Wilkinson, D. G. The EphA4 and EphB1 receptor tyrosine kinases and ephrin-B2 ligand regulate targeted migration of branchial neural crest cells. Curr. Biol. 7, 561–570 (1997).
Wang, H. U. & Anderson, D. J. Eph family transmembrane ligands can mediate repulsive guidance of trunk neural crest migration and motor axon outgrowth. Neuron 18, 383–396 (1997).
Ono, K., Tomasiewicz, H., Magnuson, T. & Rutishauser, U. N-CAM mutation inhibits tangential neuronal migration and is phenocopied by enzymatic removal of polysialic acid. Neuron 13, 595–609 (1994).
Rousselot, P., Lois, C. & Alvarez-Buylla, A. Embryonic (PSA) N-CAM reveals chains of migrating neuroblasts between the lateral ventricle and the olfactory bulb of adult mice. J. Comp. Neurol. 351, 51–61 (1995).
Serafini, T.et al. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell 87, 1001–1014 (1996).
Fazeli, A.et al. Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc) gene. Nature 386, 796–804 (1997).
Ackerman, S. L.et al. The mouse rostral cerebellar malformation gene encodes an UNC-5-like protein. Nature 386, 838–842 (1997).
Hedgecock, E. M., Culotti, J. G. & Hall, D. H. The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron 4, 61–85 (1990).
Rakic, P. Principles of neural migration. Experientia 46, 882–891 (1990).
Zallen, J. A., Yi, B. A. & Bargmann, C. I. The conserved immunoglobulin superfamily member SAX-3/Robo directs multiple aspects of axon guidance in C. elegans. Cell 92, 217–227 (1998).
Zhu, Y., Li, H. S., Zhou, L., Wu, J. Y. & Rao, Y. Cellular and molecular guidance of GABAergic neuronal migration from an extracortical origin to the neocortex. Neuron(in the press).
Acknowledgements
We thank W. Yuan and D. Ornitz for mouse slit cDNAs; J. Xu, Q. Wang and L. Zhou for help with in situ hybridization; W. Gan and J. Lichtman for help with confocal imaging and analysis; J.Brunstrom and A. Pearlman for discussions; C. S. Goodman and M. Tessier-Lavigne for comments; NIH, NSFC and SCST for support; and the John Merck Fund, NSFC and the Leukemia Society of America for scholar awards (to Y.R. and J.Y.W.).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wu, W., Wong, K., Chen, Jh. et al. Directional guidance of neuronal migration in the olfactory system by the protein Slit. Nature 400, 331–336 (1999). https://doi.org/10.1038/22477
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/22477
This article is cited by
-
Slit-Robo expression in the leech nervous system: insights into eyespot evolution
Cell & Bioscience (2023)
-
Horizontal gaze palsy with progressive scoliosis: a case report with magnetic resonance tractography and electrophysiological study
BMC Neurology (2018)
-
Desulfation of Heparan Sulfate by Sulf1 and Sulf2 Is Required for Corticospinal Tract Formation
Scientific Reports (2017)
-
How Schwann cells facilitate cancer progression in nerves
Cellular and Molecular Life Sciences (2017)
-
srGAP1 mediates the migration inhibition effect of Slit2-Robo1 in colorectal cancer
Journal of Experimental & Clinical Cancer Research (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.