Abstract
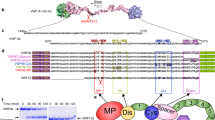
Rapid and controlled clot formation is achieved through sequential activation of circulating serine proteinase precursors on phosphatidylserine-rich procoagulant membranes of activated platelets and endothelial cells1. The homologous complexes Xase and prothrombinase, each consisting of an active proteinase and a non-enzymatic cofactor, perform critical steps within this coagulation cascade. The activated cofactors VIIIa and Va, highly specific for their cognate proteinases, are each derived from precursors with the same A1-A2-B-A3-C1-C2 architecture2. Membrane binding is mediated by the C2 domains of both cofactors. Here we report two crystal structures of the C2 domain of human factor Va. The conserved β-barrel framework provides a scaffold for three protruding loops, one of which adopts markedly different conformations in the two crystal forms. We propose a mechanism of calcium-independent, stereospecific binding of factors Va and VIIIa to phospholipid membranes3,4, on the basis of (1) immersion of hydrophobic residues at the apices of these loops in the apolar membrane core; (2) specific interactions with phosphatidylserine head groups in the groove enclosed by these loops; and (3) favourable electrostatic contacts of basic side chains with negatively charged membrane phosphate groups.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Davie,E. W., Fujikawa,K. & Kisiel,W. The coagulation cascade: initiation, maintenance, and regulation. Biochemistry 30, 10363–10370 (1991).
Kane,W. H. & Davie,E. W. Blood coagulation factors V and VIII: structural and functional similarities and their relationship to hemorrhagic and thrombotic disorders. Blood 71, 539–555 (1988).
Ortel,T. L., Devore-Carter,D., Quinn-Allen,M. A. & Kane,W. H. Deletion analysis of recombinant human factor V. Evidence for a phosphatidylserine binding site in the second C-type domain. J. Biol. Chem. 267, 4189–4198 (1992).
Comfurius,P., Smeets,E. F., Willems,G. M., Bevers,E. M. & Zwaal,R. F. A. Assembly of the prothrombinase complex on lipid vesicles depends on the stereochemical configuration of the polar headgroup of phosphatidylserine. Biochemistry 33, 10319–10324 (1994).
Stenflo,J. Contributions of Gla and EGF-like domains to the function of vitamin K-dependent coagulation factors. Crit. Rev. Eukaryot. Gene Expression 9, 59–88 (1999).
Zwaal,R. F. A., Comfurius,P. & Bevers,E. M. Lipid–protein interactions in blood coagulation. Biochim. Biophys. Acta 1376, 433–453 (1998).
Gilbert,G. E. & Drinkwater,D. Specific membrane binding of factor VIII is mediated by O-phospho-L-serine, a moiety of phosphatidylserine. Biochemistry 32, 9577–9585 (1993).
Nesheim,M. E., Taswell,J. B. & Mann,K. G. The contribution of bovine factor V and factor Va to the activity of prothrombinase. J. Biol. Chem. 254, 10952–10962 (1993).
Villoutreix,B. O., Bucher,P., Hofmann,K., Baumgartner,S. & Dahlbäck,B. Molecular models for the two discoidin domains of human blood coagulation factor V. J. Mol. Modelling 4, 268–275 (1998).
Pellequer,J. L., Gale,A. J., Griffin,J. H. & Getzoff,E. D. Homology models of the C domains of blood coagulation factors V and VIII—a proposed membrane binding mode for FV and FVIII C2 domains. Blood Cells, Molecules and Diseases 24, 448–461 (1998).
Kim,S. W. et al. Identification of functionally important amino acid residues within the C2 domain of human factor V using alanine scanning mutagenesis. Biochemistry (in the press).
Ortel,T. L. et al. Localization of functionally important epitopes within the second C-type domain of coagulation factor V using recombinant chimeras. J. Biol. Chem. 269, 15898–15905 (1994).
Ortel,T. L. et al. Inhibitory anti-factor V antibodies bind to the factor V C2 domain and are associated with hemorrhagic manifestations. Blood 91, 4188–4196 (1998).
Rosing,J., Bakker,H. M., Thomassen,M. C., Hemker,H. C. & Tans,G. Characterization of two forms of human factor Va with different cofactor activities. J. Biol. Chem. 268, 21130–21136 (1993).
Koppaka,V., Talbot,W. F., Zhai,X. & Lentz,B. R. Roles of factor Va heavy and light chains in protein and lipid rearrangements associated with the formation of a bovine factor Va-membrane complex. Biophys. J. 73, 2638–2652 (1997).
Ito,N., Phillips,S. E. V., Yadav,K. D. S. & Knowles,P. F. Crystal structure of a free radical enzyme, galactose oxidase. J. Mol. Biol. 238, 794–814 (1994).
Baumgartner,S., Hofmann,K., Chiquetehrismann,R. & Bucher,P. The discoidin domain family revisited—new members from prokaryotes and a homology-based fold prediction. Protein Sci. 7, 1626–1631 (1998).
Krishnaswamy,S. & Mann,K. G. The binding of factor Va to phospholipid vesicles. J. Biol. Chem. 263, 5714–5723 (1988).
Swairjo,M. A., Concha,N. O., Kaetzel,M. A., Dedman,J. R. & Seaton,B. A. Ca2+-bridging mechanism and phospholipid head group recognition in the membrane-binding protein annexin V. Nature Struct. Biol. 2, 968–974 (1995).
Gerads,I., Govers-Riemslag,J. W. P., Tans,G., Zwaal,R. F. A. & Rosing,J. Prothrombin activation on membranes with anionic lipids containing phosphate, sulfate, and/or carboxyl groups. J. Biol. Chem. 29, 7967–7974 (1990).
Rosing,J., Speijer,H. & Zwaal,R. F. A. Prothrombin activation on phospholipid membranes with positive electrostatic potential. Biochemistry 27, 8–11 (1988).
Cutsforth,G. A. et al. Insights into the complex association of bovine factor Va with acidic-lipid-containing synthetic membranes. Biophys. J. 70, 2938–2949 (1996).
Kalafatis,M., Rand,M. D. & Mann,K. G. Factor Va–membrane interaction is mediated by two regions located on the light chain of the cofactor. Biochemistry 33, 486–493 (1994).
Gilbert,G. E., Furie,B. C. & Furie,B. Binding of human factor VIII to phospholipid vesicles. J. Biol. Chem. 265, 815–822 (1990).
Leslie,A. in Crystallographic Computing V (eds Moras, D., Podjarny, A. D. & Thierry, J. C.) 27–38 (Oxford Univ. Press, 1991).
Collaborative Computational Project No. 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994).
Knight,S. Ribulose 1,5-biphosphate carboxylase/oxygenase—a structural study. (PhD Thesis, Swedish Univ. Agricultural Sciences, Uppsala, 1989).
Brünger,A. T. X-PLOR Version 3.1: A System for X-ray Crystallography and NMR (Yale Univ. Press, New Haven, 1993).
Navaza,J. An automated package for molecular replacement. Acta Crystallogr. A 50, 157–163 (1994).
Nicholls,A., Sharp,K. A. & Honig,B. Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins Struct. Funct. Genet. 11, 281–296 (1991).
Acknowledgements
We thank D. Grosse and M. Renatus for initial crystallization trials. W.B. is supported by the Biomed. and Training and Mobility Programs of the EU, by the HFSP and by the SFB469; M.A.Q.-A. and W.H.K. by the NIH; T.L.O., a Pew Scholar, by the March of Dimes Birth Defects Foundation; and S.M.-R. by PraxisXXI, FCT, Portugal.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Macedo-Ribeiro, S., Bode, W., Huber, R. et al. Crystal structures of the membrane-binding C2 domain of human coagulation factor V. Nature 402, 434–439 (1999). https://doi.org/10.1038/46594
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/46594
This article is cited by
-
The role of phosphatidylserine on the membrane in immunity and blood coagulation
Biomarker Research (2022)
-
Crystal structure and biochemical analyses reveal Beclin 1 as a novel membrane binding protein
Cell Research (2012)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.