Abstract
Purpose
To compare the short-term visual and morphological results of intravitreal triamcinolone acetonide vsintravitreal bevacizumab for eyes with macular oedema secondary to branch retinal vein occlusion (BRVO).
Design
Retrospective interventional consecutive case series.
Methods
We reviewed the clinical records of 29 patients (29 eyes) who had macular oedema due to BRVO with minimum follow-up of 6 months. A total of 16 patients were treated with intravitreal injection of 4 mg/0.1ml triamcinolone acetonide. The other 13 patients received intravitreal bevacizumab of 1.25 mg in 0.05 ml. Baseline visual acuity, macular thickness, and intraocular pressure were recorded. Final visual acuity, final macular thickness, intraocular pressure, and adverse events were also recorded throughout the follow-up.
Results
All patients completed at least 6 months of follow-up. There were significant improvement in visual acuity and showed significant macular oedema decrease in optical coherence tomography examination in both the two groups postoperatively. However the therapeutic effects showed no statistically significant difference between these two groups with regard to visual results (F=6.012, P=0.083) and macular thickness decline (F=0.007, P=0.570). Seven eyes developed recurrent macular oedema and received reinjections of triamcinolone acetonide or bevacizumab.
Conclusion
These short-term results indicate that intravitreal injection of triamcinolone acetonide or bevacizumab can both improve visual acuity and decrease macular oedema temporarily in eyes with BRVO. However, the therapeutic effects of intravitreal triamcinolone acetonide showed no significant differences compared with intravitreal bevacizumab with regard to anatomical and functional outcomes but seemed to cause more adverse events than bevacizumab.
Similar content being viewed by others
Introduction
Branch retinal vein occlusion (BRVO) is a common retinal vascular disease seen most frequently in individuals who are older than 50 years.1, 2 It affects males and females equally, and the most common site is at the superotemporal quadrant.3, 4, 5, 6 Patients often complain of sudden onset of blurred vision or visual field defect. The fundus shows intraretinal haemorrhage, retinal oedema, and often cotton-wool spots in a sector of retina drained by the affected vein. The vision-limiting complications include macular oedema, retinal capillary non-perfusion, and vitreous haemorrhage from neovascularisation.7, 8, 9 Macular oedema is the major cause of visual disturbance in BRVO, occurring in about 60% of cases.10 The degree of macular involvement determines the level of visual impairment.
At present, macular grid laser photocoagulation has been the only established treatment modality for macular oedema arising from BRVO, but the visual acuity improvement is often very limited (average improvement in vision of 1.33 Snellen lines).2 It may also be associated with several complications, including submacular fibrosis,11 visual-field sensitivity deterioration,12, 13 enlargement of laser scar,14 and choroidal neovascularisation.15
This small and insufficient response to laser therapy led to the search for other new therapeutic options. Recently, various medical and surgical strategies have been tried by many physicians to treat macular oedema secondary to BRVO. Several studies have demonstrated the usefulness of intravitreal injection of triamcinolone acetonide16, 17, 18 and of anti-vascular endothelial growth factor (anti-VEGF) agents, such as bevacizumab19, 20 and ranibizumab,21 in dealing with macular oedema due to BRVO. These treatment modalities have been reported to be associated with short-term promising anatomical and functional improvement in some patients with macular oedema due to BRVO. In view of these promising preliminary studies, we present a retrospective review of data to compare the tomographical and visual outcomes after intravitreal injection of triamcinolone acetonide vs bevacizumab in the treatment of macular oedema secondary to BRVO.
Materials and methods
Data collection
We conducted a retrospective chart review of 29 eyes of 29 consecutive patients with macular oedema due to BRVO. Medical records were reviewed for all patients with BRVO and macular oedema at the Department of Ophthalmology, Kaohsiung Medical University, Chung-Ho Memorial Hospital between January 2004 and March 2008. 16 eyes accepted 4-mg/0.1-ml intravitreal triamcinolone acetonide and 13 eyes were given 1.25-mg/0.05-ml intravitreal bevacizumab. Patients accepting intravitreal triamcinolone constituted the ‘ITA’ group, and those receiving intravitreal bevacizumab constituted the ‘IBe’ group. All patients in this consecutive series completed a minimum of 6 months of follow-up.
No cataract surgery was performed before, in combination with, or after, the intravitreal injection. The following data were collected: ophthalmic and medical history; best-corrected visual acuity (the best-corrected visual acuity was determined from Snellen chart and converted to the logarithm of minimal angle of resolution (LogMAR) equivalents before the statistical analysis; counting fingers and hand movements at 1 m were converted to 1.6 and 1.9, respectively); slit-lamp examination of the anterior segment; intraocular pressure (IOP) measurement (Full auto Tonometer TX-F; Canon, New York, NY, USA); dilated fundus examination with indirect ophthalmoscopy and Goldmann 3-mirror contact lens; and central macular thickness quantitatively measured by optical coherence tomography (Stratus OCT III Model 3000; Zeiss Humphery, New York, NY, USA). In each patient, the same instrument (optical coherence tomography) was used throughout with 6-mm radial lines employed. The 1-mm mean central retinal thickness was obtained using retinal thickness map for our analysis.
The patients were initially followed up at the first week post-injection, and twice at two-weekly intervals, then at routine monthly intervals. Repeated injections of ITA or IBe were carried out as needed, based on the recurrence of macular oedema on OCT or deterioration in visual acuity. The interval of follow-up examinations was increased to longer periods once the macular oedema resolved, or the visual acuity became stable or improved.
The main outcome measures were best-corrected visual acuity, macular thickness assessed with optical coherence tomography, and postoperative complications.
Surgical procedure
Informed consent was obtained after discussing extensively with each patient about the benefits, risks, and possible side effects of the two drugs. All intravitreal injections were carried out according to a standard protocol at the Department of Ophthalmology, Kaohsiung Medical University, Chung-Ho Memorial Hospital. The intravitreal injection of triamcinolone acetonide or bevacizumab was carried out under aseptic conditions in the operating room with an operation microscope. After obtaining informed consent, the affected eye was prepared in a standard fashion using a drop of proparacaine hydrochloride (0.5%) ophthalmic solution to the ocular surface for topical anaesthesia, followed by topical application of 5% povidone-iodine (Saint-iodine; Patron, Gangshan, Taiwan) for periocular area, lids, eyelashes, and conjunctiva before the intravitreal injection. Then, the patient was completely draped. An eyelid speculum was used to stabilize the eyelids. A paracentesis in the anterior chamber was performed and 0.1 ml of aqueous fluid was aspirated by 26-G needle with a 1.0-ml tuberculin syringe to decrease the volume of the eye, thereby avoiding a rise in IOP. 4-mg (0.1 ml) crystalline triamcinolone acetonide (Kenacort-A; Bristol-Myers Squibb, Taipei, Taiwan) or 1.25-mg (0.05 ml) bevacizumab (Avastin, Genentech, San Francisco, CA, USA/Hoffmann La Roche, Basel, Switzerland) was injected into the vitreous cavity via the pars plana 3.5–4 mm posterior to the limbus using a sharp 27-G needle. The inferior pars plana was selected for injection to minimize postoperative floaters because the injected triamcinolone acetonide rapidly deposits to dependent areas of the vitreous cavity after treatment. After the injection, a topical antibiotic was applied and eyes were patched for several hours. After surgery, patients were instructed to administer topical antibiotic eyedrop (Tobramycin-Tobrex; Alcon, Belgium, China) four times daily, for 3 days.
Statistical analysis
Baseline demographic and clinical parameters were compared between the two groups using independent-samples t-test for continuous variables and χ2-tests for categorical variables. The visual acuity was converted to LogMAR for statistical analysis. Visual acuity and macular thickness at the baseline and final follow-up visits were summarized using mean±SD. The change in the visual acuity and macular thickness during follow-up was calculated for each case, and the mean change across all cases was compared between the ITA and IBe groups. Comparisons of change, during follow-up, using between group comparisons at the final visit utilized independent-samples t-test. In addition, paired t-tests were run to compare differences between baseline and final follow-up data within each treatment group (for visual acuity and macular thickness).
Statistical analyses employed commercially available software (SPSS, version 12.0; SPSS Inc., Chicago, IL, USA). The level of statistical significance was set at two-tailed P-value <0.05.
Results
Baseline characteristics
A total of 16 cases treated with triamcinolone acetonide between January 2004 and November 2007 and 13 cases treated with bevacizumab between August 2006 and March 2008 were eligible for analysis. The average age of the ITA group was 55.75±10.00 years and the average age of the IBe group was 55.38±13.14 years. The average follow-up times were 265.69±78.78 days (ranging from 183 to 398 days) in the ITA group and 223.23±41.63 days (ranging from 185 to 303 days) in the IBe group, respectively. All patients completed, at least, 6 months of follow-up. Of the 16 eyes in the ITA group, focal laser photocoagulation was carried out on seven cases (cases 1, 2, 3, 4, 11, 13, and 14) during the follow-up periods to prevent neovascular sequelae. In all, 8 out of 13 eyes (cases 2, 4, 6, 7, 8,10, 11, and 12) in the IBe group had also been treated with focal laser during the follow-up duration to avoid neovascular complications. One patient received one-time re-injection and another patient received two-time re-injections of triamcinolone acetonide between baseline and the final follow-up. Four patients received re-injection once and one patient received re-injections twice of bevacizumab within the follow-up period. The baseline characteristics by group are matched and listed in Table 1; there were no statistically significant differences between the two treatment groups with regard to patient age, sex, follow-up period, baseline visual acuity, and baseline retinal thickness.
Outcome measures
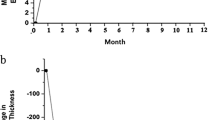
In the ITA group, visual acuity measurements improved significantly (P<0.001) from 0.77±0.45 LogMAR preoperatively to a final best-corrected visual acuity of 0.39±0.42 LogMAR postoperatively. All eyes showed visual acuity improvement during the follow-up period compared with the baseline measurement of the study. Measured in Snellen lines, 11 eyes (68.75%) showed an improvement by at least two Snellen lines or more (Table 2). In the IBe group, visual acuity measurements also improved significantly (P<0.001) from 0.99±0.48 LogMAR preoperatively to a final best-corrected visual acuity of 0.35±0.32 LogMAR postoperatively. Twelve eyes (92.31%) showed visual acuity improvement and one eye (7.69%) remained the same during the follow-up period compared with the baseline measurement of the study. Measured in Snellen lines, nine eyes (69.23%) showed an improvement by at least two Snellen lines or more (Table 3). However, between-group comparisons with respect to change in visual acuity showed no statistically significant differences (F=6.012, P=0.083) (Figure 1a).
Comparison between the intravitreal triamcinolone (ITA) group and the intravitreal bevacizumab (IBe) group for changes in mean visual acuity (a) and mean central foveal thickness (b) postoperatively. Negative values represent an increase in visual acuity and a reduction in the central foveal thickness. For the ITA group and the IBe group, no significant difference in visual acuity improvement or central foveal thickness reduction was noted between the two groups. (P=0.083 in visual acuity improvement and P=0.570 in central foveal thickness reduction).
The decline of cotton-wool spots, retinal haemorrhage, and macular oedema were noted in all the cases in these two groups during the examination of the fundus, that is after intravitreal injection of triamcinolone acetonide or bevacizumab, and the fluorescein angiography showed vascular leakage decrease postoperatively in every case of these two groups.
The optical coherence tomography examination of the ITA group demonstrated clinical improvement in macular oedema (P<0.001) postoperatively. The preinjection foveal thickness ranged from 253 to 782 μm (mean, 533.63±163.75 μm). The final foveal thickness ranged from 164 to 421 μm (mean, 254.00±80.06 μm) with an average decrease of 48.87% in the foveal thickness (Table 2). In the IBe group, the foveal thickness measured by optical coherence tomography between baseline and postoperative data also show significant resolution (P<0.001). The preinjection foveal thickness ranged from 295 to 971 μm (mean, 538.85±189.80 μm). The final foveal thickness ranged from 167 to 277 μm (mean, 222.00±36.38 μm) with an average decrease of 54.29% in the foveal thickness (Table 3). However, changes in the foveal thickness did not statistically significantly differ between these two treatment groups (F=0.007, P=0.570) (Figure 1b).
Recurrence of macular oedema and a concomitant decrease in visual acuity occurred in two cases of the ITA group (cases 4 and 13) at an average of 201.33±37.07 days (ranging from 172 to 243 days) postoperatively, and in five cases of the IBe group (cases 1, 4, 8, 9, and 13) with an average of 148.17±30.94 days (ranging from 98 to 175 days) after intravitreal injection. Subsequently, one patient (case 4) in the ITA group accepted a second injection and another patient (case 13) received three separate injections of triamcinolone acetonide. In the IBe group, four cases received re-injection once and one case (case 8) received re-injections of bevacizumab twice. After that, the macular oedema in all seven patients subsided and visual acuity improved again in two patients (cases 4 and 13) of the ITA group and in four patients (cases 4, 8, 9, and 13) of the IBe group, respectively.
Three patients (18.75%) in the ITA group had ocular hypertension (IOP ≧ 22 mmHg) postoperatively. All three patients (cases 3, 8, and 13) could be controlled to a normal range by topical anti-glaucomatous agents. There was no case with increase in IOP after intravitreal injection in the IBe group.
Adverse events
Excluding elevated IOP, no obvious complication was noted in the ITA group postoperatively. No increase in IOP and no cataract progression were observed in the IBe group postoperatively. No serious side effects were observed throughout the study. No systemic or serious drug-related adverse events were observed. Both treatment procedures were well tolerated and none of the patients showed any clinical evidence of local adverse events such as inflammation, uveitis, postoperative endophthalmitis, retinal detachment, or ocular toxicity, and no systemic complications like thrombo-embolic events were encountered in any case during the follow-up period.
Discussion
As far as we know, and according to Medline searches, this study is the first retrospective data to compare intravitreal triamcinolone with bevacizumab for the management of patients with macular oedema secondary to BRVO. Best-corrected visual acuity and foveal thickness were used to evaluate disease control. In this study, intravitreal injection of triamcinolone acetonide provided similar therapeutic efficacy in patients with macular oedema due to BRVO compared with intravitreal injection of bevacizumab in the short-term period.
These patients of the ITA group experienced a significant increase in visual acuity (P<0.001) from 0.77±0.45 LogMAR preoperatively to a final best postoperative visual acuity of 0.39±0.42 LogMAR postoperatively, and those in the IBe group also had significant visual acuity improvement (P<0.001) from 0.99±0.48 LogMAR preoperatively to a final best postoperative visual acuity of 0.35±0.32 LogMAR postoperatively. We observed a significant improvement in the central foveal thickness after intravitreal injection of intravitreal triamcinolone (average decrease percentage: 48.87%, P<0.001) or bevacizumab (average decrease percentage: 54.29%, P<0.001). From our results, both intravitreal triamcinolone acetonide and bevacizumab have been demonstrated to decrease vascular leakage and improve the functional and tomographical outcomes in patients with macula oedema associated with BRVO. However, the changes in visual acuity and the foveal thickness did not show any significant difference between the ITA group and the IBe group (P=0.083 in visual acuity improvement and P=0.570 in the foveal thickness decrease). Therefore, the overall results of our study suggest that intravitreal injection of triamcinolone acetonide may have the same beneficial effects on vision and macular remodelling, as intravitreal injection of bevacizumab for the short-term management of macular oedema associated with BRVO.
Intravitreal triamcinolone acetonide has become an increasingly popular treatment for macular oedema associated with various aetiologies.22, 23, 24, 25 The mechanism of action of corticosteroids for macular oedema in BRVO is still under investigation, but it is postulated that anti-inflammation, VEGF downregulation and anti-permeability functions of corticosteroid were the major roles for its effect.26, 27, 28, 29 In a retrospective chart review of 13 eyes of 13 patients, Cekiç et al16 reported that after administration of 4-mg intravitreal triamcinolone acetonide for macular oedema due to BRVO, all 13 eyes showed biomicroscopic improvement in cystoid macular oedema. Compared with baseline, 7 eyes had visual improvement (range 2–6 Snellen lines), remained the same in 4 eyes (range 0–1 Snellen lines), and worsened in 2 eyes (range −1 to −4 Snellen lines) at the end of follow-up. Krepler et al17 evaluated the therapeutic effect of 4-mg intravitreal triamcinolone acetonide for nine patients with macular oedema secondary to BRVO. This prospective case series study revealed a significant functional benefit, as well as anatomical improvement in macular oedema. These results were similar to the findings in our study.
Intravitreal bevacizumab was first used by Pai et al30 as a treatment for macular oedema related to BRVO. Consequently, there have been other reports of short-term beneficial effect of intravitreal bevacizumab to treat macular oedema secondary to retinal vascular disease, including central retinal vein occlusion31, 32 and diabetic retinopathy.33, 34 Gündüz et al20 reported a dramatic improvement in the visual acuity with significant macular thickness reduction after intravitreal bevacizumab injections (1.25 mg/0.05 ml) for patients with BRVO. Jaissle et al35 demonstrated for the first time a significant long-term effect of intravitreal bevacizumab(1.25 mg/0.05 ml) for the macular oedema due to BRVO. Their study showed a 39% reduction of the median central retinal thickness and a decrease of 0.3 LogMAR in median visual acuity at 48 weeks. More recently, the result of a prospective clinical trial carried out by Prager et al36 showed that in the BRVO group (n=18) after intravitreal bevacizumab (1 mg/0.04 ml), visual acuity increased from 55 ETDRS letters at baseline to 73 ETDRS letters (P<0.001) and central retinal thickness decreased significantly by 241 μm (P<0.001) after 1 year of follow-up. In our case series, we observed significant improvement in visual acuity and central foveal thickness decrease after injection of 1.25-mg bevacizumab.
Recent clinical and experimental studies have demonstrated that intravitreal triamcinolone acetonide or bevacizumab have shown no evidence of any toxicity to the retina.37, 38, 39, 40, 41, 42, 43 Intravitreal triamcinolone acetonide treatment may lead to complications, such as ocular hypertension, cataract progression, retinal detachment, intraocular haemorrhage, and infectious endophthalmitis. The most common side effect reported after intravitreal injection of triamcinolone acetonide is the risk of IOP elevation.16, 44, 45 In this study, 3 out of 16 BRVO patients without preexisting glaucoma developed steroid-induced elevated IOP after intravitreal triamcinolone acetonide injection and all were successfully controlled with topical anti-glaucomatous eyedrops. In the IBe group, the IOP was normal even after intravitreal injection. It is important to point out that intravitreal triamcinolone acetonide has a higher risk of short-term elevation of IOP than intravitreal bevacizumab. In addition, the incidence of development or progression of cataract may increase with intravitreal triamcinolone acetonide treatments.16, 23, 46 In our study, no case in the ITA group suffered from cataract formation during the follow-up. To avoid the above complications mentioned in triamcinolone acetonide injection, intravitreal bevacizumab may be an attractive alternative therapeutic option for phakic patients and steroid responders because it provides visual acuity improvement and restoration of retinal anatomy without the side effects of ocular hypertension and cataract progression, and the absence of any inflammation suggests that even repeated injections are well tolerated. Additional injection-related complications reported in other studies, such as conjunctival ulceration,47 retinal detachment,48 infectious or non-infectious endophthalmitis,48, 49, 50 were not observed in our study.
The effects of intravitreal triamcinolone acetonide or bevacizumab have been reported to be temporary and might be related to their clearance from the eye. Yepremyan et al51 demonstrated that 8 out of 12 eyes with BRVO developed recurrent cystoid macular oedema at an average of 5.5 months after initial intravitreal triamcinolone acetonide injection and required additional intervention during the follow-up period, thus indicating that the therapeutic effect of triamcinolone acetonide probably persisted for 5.5 months with BRVO. Gündüz20 et al reported that the duration of intravitreal bevacizumab effect appears to be limited to 3.4 months for most patients with BRVO. Relapse of macular oedema at an average of 12 weeks after intravitreal bevacizumab has been demonstrated by Jaissle et al.35 Other reports also disclosed similar periods ranging from 2 to 3 months from the last intravitreal bevacizumab to recurrence of macular oedema.18, 19, 30 This is similar to our finding that the mean time for recurrence of macular oedema was 201.33±37.07 days in the ITA group and 148.17±30.94 days in the IBe group, respectively. According to the above results, intravitreal triamcinolone acetonide seems to persist longer than intravitreal bevacizumab, which may allow a more prolonged inhibition of VEGF and reduce the numbers of re-injections. Another superiority of triamcinolone acetonide is the relatively low price compared with bevacizumab. In our country, bevacizumab is far more expensive than triamcinolone acetonide.
Some limitations are inherent in our study, such as the small sample size in both the ITA and the IBe group, the retrospective study design, limited duration of follow-up, non-standardized guidelines for repeated injection and non-randomized trial. Large prospective, randomized clinical trials are necessary to compare the long-term efficacy and safety of intravitreal triamcinolone acetonide with intravitreal bevacizumab for patients with macular oedema associated with BRVO.
Conclusion
In conclusion, intravitreal injection of 4-mg triamcinolone acetonide appears to provide the same short-term advantages as 1.25-mg intravitreal bevacizumab for the management of patients with macular oedema secondary to BRVO. Intraocular steroid or anti-VEGF agents can cause rapid resolution of macular oedema and visual acuity improvement, but the effects are not permanent. Repeated injections may be necessary before the complete resolution of macular oedema in some patients. The potential benefits for intravitreal triamcinolone acetonide to manage macular oedema associated with BRVO are the relatively low cost and longer half-life. However, the benefits of triamcinolone acetonide have been complicated by the well-known adverse events such as increased rates of cataract formation and elevated IOP. The merit of intravitreal bevacizumab is the lack of serious adverse side effects, but the expensive price is its shortcoming. Further prospective investigations in larger populations and longer follow-up duration with appropriate control group are needed to find the best approach (either intravitreal triamcinolone acetonide or intravitreal bevacizumab) to patients with macular oedema associated with BRVO.
Conflict of interest
The authors declare no conflict of interest.
References
Mitchell P, Smith W, Chang A . Prevalence and associations of retinal vein occlusion in Australia. The Blue Mountains Eye Study. Arch Ophthalmol 1996; 114 (10): 1243–1247.
Argon laser photocoagulation for macular edema in branch vein occlusion. The Branch Vein Occlusion Study Group. Am J Ophthalmol 1984; 98 (3): 271–282.
Becker B, Post LT Jr . Retinal vein occlusion. Clinical and experimental observations. Am J Ophthalmol 1951; 34 (5:1): 677–686.
Blankenship GW, Okun E . Retinal tributary vein occlusion. History and management by photocoagulation. Arch Ophthalmol 1973; 89 (5): 363–368.
Gutman FA, Zegarra H . The natural course of temporal retinal branch vein occlusion. Trans Am Acad Ophthalmol Otolaryngol 1974; 78 (2): OP178–OP192.
Christoffersen NL, Larsen M . Pathophysiology and hemodynamics of branch retinal vein occlusion. Ophthalmology 1999; 106 (11): 2054–2062.
Gutman FA, Zegarra H . Macular edema secondary to occlusion of the retinal veins. Surv Ophthalmol 1984; 28 (Suppl): 462–470.
Finkelstein D . Ischemic macular edema. Recognition and favorable natural history in branch vein occlusion. Arch Ophthalmol 1992; 110 (10): 1427–1434.
McAllister IL, Douglas JP, Constable IJ, Yu DY . Laser-induced chorioretinal venous anastomosis for non-ischemic central retinal vein occlusion: evaluation of the complications and their risk factors. Am J Ophthalmol 1998; 126 (2): 219–229.
Greer DV, Constable IJ, Cooper RL . Macular oedema and retinal branch vein occlusion. Aust J Ophthalmol 1980; 8 (3): 207–209.
Han DP, Mieler WF, Burton TC . Submacular fibrosis after photocoagulation for diabetic macular edema. Am J Ophthalmol 1992; 113 (5): 513–521.
Striph GG, Hart WM Jr, Olk RJ . Modified grid laser photocoagulation for diabetic macular edema. The effect on the central visual field. Ophthalmology 1988; 95 (12): 1673–1679.
Okuyama M, Okisaka S . Automatic static threshold perimetry is useful for estimating the effects of laser photocoagulation on diabetic maculopathy. Ophthalmic Res 1998; 30 (4): 207–215.
Schatz H, Madeira D, McDonald HR, Johnson RN . Progressive enlargement of laser scars following grid laser photocoagulation for diffuse diabetic macular edema. Arch Ophthalmol 1991; 109 (11): 1549–1551.
Lewis H, Schachat AP, Haimann MH, Haller JA, Quinlan P, von Fricken MA et al. Choroidal neovascularization after laser photocoagulation for diabetic macular edema. Ophthalmology 1990; 97 (4): 503–510; discussion 510–501.
Cekiç O, Chang S, Tseng JJ, Barile GR, Del Priore LV, Weissman H et al. Intravitreal triamcinolone injection for treatment of macular edema secondary to branch retinal vein occlusion. Retina 2005; 25 (7): 851–855.
Krepler K, Ergun E, Sacu S, Richter-Müksch S, Wagner J, Stur M et al. Intravitreal triamcinolone acetonide in patients with macular oedema due to branch retinal vein occlusion: a pilot study. Acta Ophthalmol Scand 2005; 83 (5): 600–604.
Chen SD, Sundaram V, Lochhead J, Patel CK . Intravitreal triamcinolone for the treatment of ischemic macular edema associated with branch retinal vein occlusion. Am J Ophthalmol 2006; 141 (5): 876–883.
Rabena MD, Pieramici DJ, Castellarin AA, Nasir MA, Avery RL . Intravitreal bevacizumab (Avastin) in the treatment of macular edema secondary to branch retinal vein occlusion. Retina 2007; 27 (4): 419–425.
Gündüz K, Bakri SJ . Intravitreal bevacizumab for macular oedema secondary to branch retinal vein occlusion. Eye 2008; 22 (9): 1168–1171.
Campochiaro PA, Hafiz G, Shah SM, Nguyen QD, Ying H, Do DV et al. Ranibizumab for macular edema due to retinal vein occlusions: implication of VEGF as a critical stimulator. Mol Ther 2008; 16 (4): 791–799.
Antcliff RJ, Spalton DJ, Stanford MR, Graham EM, ffytche TJ, Marshall J . Intravitreal triamcinolone for uveitic cystoid macular edema: an optical coherence tomography study. Ophthalmology 2001; 108 (4): 765–772.
Martidis A, Duker JS, Greenberg PB, Rogers AH, Puliafito CA, Reichel E et al. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology 2002; 109 (5): 920–927.
Andrade RE, Muccioli C, Farah ME, Nussenblatt RB, Belfort R Jr . Intravitreal triamcinolone in the treatment of serous retinal detachment in Vogt–Koyanagi–Harada syndrome. Am J Ophthalmol 2004; 137 (3): 572–574.
Goff MJ, Jumper JM, Yang SS, Fu AD, Johnson RN, McDonald HR et al. Intravitreal triamcinolone acetonide treatment of macular edema associated with central retinal vein occlusion. Retina 2006; 26 (8): 896–901.
Vinores SA, Youssri AI, Luna JD, Chen YS, Bhargave S, Vinores MA et al. Upregulation of vascular endothelial growth factor in ischemic and non-ischemic human and experimental retinal disease. Histol Histopathol 1997; 12 (1): 99–109.
Fischer S, Renz D, Schaper W, Karliczek GF . In vitro effects of dexamethasone on hypoxia-induced hyperpermeability and expression of vascular endothelial growth factor. Eur J Pharmacol 2001; 411 (3): 231–243.
Noma H, Funatsu H, Yamasaki M, Tsukamoto H, Mimura T, Sone T et al. Pathogenesis of macular edema with branch retinal vein occlusion and intraocular levels of vascular endothelial growth factor and interleukin-6. Am J Ophthalmol 2005; 140 (2): 256–261.
Noma H, Minamoto A, Funatsu H, Tsukamoto H, Nakano K, Yamashita H et al. Intravitreal levels of vascular endothelial growth factor and interleukin-6 are correlated with macular edema in branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol 2006; 244 (3): 309–315.
Pai SA, Shetty R, Vijayan PB, Venkatasubramaniam G, Yadav NK, Shetty BK et al. Clinical, anatomic, and electrophysiologic evaluation following intravitreal bevacizumab for macular edema in retinal vein occlusion. Am J Ophthalmol 2007; 143 (4): 601–606.
Iturralde D, Spaide RF, Meyerle CB, Klancnik JM, Yannuzzi LA, Fisher YL et al. Intravitreal bevacizumab (avastin) treatment of macular edema in central retinal vein occlusion: a short-term study. Retina 2006; 26 (3): 279–284.
Priglinger SG, Wolf AH, Kreutzer TC, Kook D, Hofer A, Strauss RW et al. Intravitreal bevacizumab injections for treatment of central retinal vein occlusion: six-month results of a prospective trial. Retina 2007; 27 (8): 1004–1012.
Haritoglou C, Kook D, Neubauer A, Wolf A, Priglinger S, Strauss R et al. Intravitreal bevacizumab (avastin) therapy for persistent diffuse diabetic macular edema. Retina 2006; 26 (9): 999–1005.
Arevalo JF, Fromow-Guerra J, Quiroz-Mercado H, Sanchez JG, Wu L, Maia M et al. Primary intravitreal bevacizumab (Avastin) for diabetic macular edema: results from the Pan-American Collaborative Retina Study Group at 6-month follow-up. Ophthalmology 2007; 114 (4): 743–750.
Jaissle GB, Leitritz M, Gelisken F, Ziemssen F, Bartz-Schmidt KU, Szurman P . One-year results after intravitreal bevacizumab therapy for macular edema secondary to branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol 2009; 247 (1): 27–33.
Prager F, Michels S, Kriechbaum K, Georgopoulos M, Funk M, Geitzenauer W et al. Intravitreal bevacizumab (Avastin) for macular oedema secondary to retinal vein occlusion: 12-month results of a prospective clinical trial. Br J Ophthalmol 2009; 93 (4): 452–456.
McCuen BW 2nd, Bessler M, Tano Y, Chandler D, Machemer R . The lack of toxicity of intravitreally administered triamcinolone acetonide. Am J Ophthalmol 1981; 91 (6): 785–788.
Hida T, Chandler D, Arena JE, Machemer R . Experimental and clinical observations of the intraocular toxicity of commercial corticosteroid preparations. Am J Ophthalmol 1986; 101 (2): 190–195.
Kivilcim M, Peyman GA, El-Dessouky ES, Kazi AA, Cheema R, Hegazy H . Retinal toxicity of triamcinolone acetonide in silicone-filled eyes. Ophthalmic Surg Lasers 2000; 31 (6): 474–478.
Luthra S, Narayanan R, Marques LE, Chwa M, Kim DW, Dong J et al. Evaluation of in vitro effects of bevacizumab (Avastin) on retinal pigment epithelial, neurosensory retinal, and microvascular endothelial cells. Retina 2006; 26 (5): 512–518.
Manzano RP, Peyman GA, Khan P, Kivilcim M . Testing intravitreal toxicity of bevacizumab (Avastin). Retina 2006; 26 (3): 257–261.
Shahar J, Avery RL, Heilweil G, Barak A, Zemel E, Lewis GP et al. Electrophysiologic and retinal penetration studies following intravitreal injection of bevacizumab (Avastin). Retina 2006; 26 (3): 262–269.
Maturi RK, Bleau LA, Wilson DL . Electrophysiologic findings after intravitreal bevacizumab (Avastin) treatment. Retina 2006; 26 (3): 270–274.
Lee H, Shah GK . Intravitreal triamcinolone as primary treatment of cystoid macular edema secondary to branch retinal vein occlusion. Retina 2005; 25 (5): 551–555.
Vasconcelos-Santos DV, Nehemy PG, Schachat AP, Nehemy MB . Secondary ocular hypertension after intravitreal injection of 4 mg of triamcinolone acetonide: incidence and risk factors. Retina 2008; 28 (4): 573–580.
Cekiç O, Chang S, Tseng JJ, Barile GR, Weissman H, Del Priore LV et al. Intravitreal triamcinolone treatment for macular edema associated with central retinal vein occlusion and hemiretinal vein occlusion. Retina 2005; 25 (7): 846–850.
Agrawal S, Agrawal J, Agrawal TP . Conjunctival ulceration following triamcinolone injection. Am J Ophthalmol 2003; 136 (3): 539–540.
Jonas JB, Spandau UH, Schlichtenbrede F . Short-term complications of intravitreal injections of triamcinolone and bevacizumab. Eye 2008; 22 (4): 590–591.
Nelson ML, Tennant MT, Sivalingam A, Regillo CD, Belmont JB, Martidis A . Infectious and presumed non-infectious endophthalmitis after intravitreal triamcinolone acetonide injection. Retina 2003; 23 (5): 686–691.
Roth DB, Chieh J, Spirn MJ, Green SN, Yarian DL, Chaudhry NA . Noninfectious endophthalmitis associated with intravitreal triamcinolone injection. Arch Ophthalmol 2003; 121 (9): 1279–1282.
Yepremyan M, Wertz FD, Tivnan T, Eversman L, Marx JL . Early treatment of cystoid macular edema secondary to branch retinal vein occlusion with intravitreal triamcinolone acetonide. Ophthalmic Surg Lasers Imaging 2005; 36 (1): 30–36.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheng, KC., Wu, WC. & Chen, KJ. Intravitreal triamcinolone acetonide vs bevacizumab for treatment of macular oedema secondary to branch retinal vein occlusion. Eye 23, 2023–2033 (2009). https://doi.org/10.1038/eye.2009.230
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2009.230
Keywords
This article is cited by
-
Results of intravitreal triamcinolone acetonide in patients with macular edema secondary to branch retinal vein occlusion
International Journal of Clinical Pharmacy (2014)
-
Visual prognostic value of photopic negative response and optical coherence tomography in central retinal vein occlusion after anti-VEGF treatment
Documenta Ophthalmologica (2013)
-
Three intravitreal bevacizumab versus two intravitreal triamcinolone injections in recent-onset branch retinal vein occlusion
Graefe's Archive for Clinical and Experimental Ophthalmology (2012)
-
Recurrence of macular edema associated with branch retinal vein occlusion after intravitreal bevacizumab
Japanese Journal of Ophthalmology (2012)
-
Intravitreal triamcinolone acetonide versus bevacizumab therapy for macular edema associated with branch retinal vein occlusion
Graefe's Archive for Clinical and Experimental Ophthalmology (2010)