Abstract
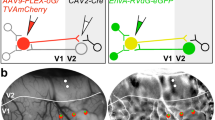
Thalamic nuclei are thought to funnel sensory information to the brain's primary cortical areas, which in turn transmit signals afresh to higher cortical areas. Here we describe a direct projection in the macaque monkey from the lateral geniculate nucleus (LGN) to the motion-selective middle temporal area (MTor V5), a cortical area not previously considered 'primary'. The constituent neurons are mostly koniocellular, send virtually no collateral axons to primary visual cortex (V1) and equal about 10% of the V1 population innervating MT. This pathway could explain the persistence of motion sensitivity in subjects following injury to V1, suggesting more generally that residual perception after damage in a primary area may arise from sparse thalamic input to 'secondary' cortical areas.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Riddoch, G. Dissociation of visual perceptions due to occipital injuries, with especial reference to appreciation of movement. Brain 40, 15–57 (1917).
Zeki, S. & ffytche, D.H. The Riddoch syndrome: insights into the neurobiology of conscious vision. Brain 121, 25–45 (1998).
Humphrey, N.K. & Weiskrantz, L. Vision in monkeys after removal of the striate cortex. Nature 215, 595–597 (1967).
Cowey, A. & Stoerig, P. Blindsight in monkeys. Nature 373, 247–249 (1995).
Stoerig, P. & Cowey, A. Blindsight in man and monkey. Brain 120, 535–559 (1997).
Newsome, W.T., Britten, K.H. & Movshon, J.A. Neuronal correlates of a perceptual decision. Nature 341, 52–54 (1989).
Salzman, C.D., Murasugi, C.M., Britten, K.H. & Newsome, W.T. Microstimulation in visual area MT: effects on direction discrimination performance. J. Neurosci. 12, 2331–2355 (1992).
Watson, J.D. et al. Area V5 of the human brain: evidence from a combined study using positron emission tomography and magnetic resonance imaging. Cereb. Cortex 3, 79–94 (1993).
Beckers, G. & Zeki, S. The consequences of inactivating areas V1 and V5 on visual motion perception. Brain 118, 49–60 (1995).
Zeki, S.M. The secondary visual areas of the monkey. Brain Res. 13, 197–226 (1969).
Cragg, B.G. The topography of the afferent projections in the circumstriate visual cortex of the monkey studied by the Nauta method. Vision Res. 9, 733–747 (1969).
Zeki, S. & Shipp, S. The functional logic of cortical connections. Nature 335, 311–317 (1988).
Felleman, D.J. & Van Essen, D.C. Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex 1, 1–47 (1991).
Yukie, M. & Iwai, E. Direct projection from the dorsal lateral geniculate nucleus to the prestriate cortex in macaque monkeys. J. Comp. Neurol. 201, 81–97 (1981).
Fries, W. The projection from the lateral geniculate nucleus to the prestriate cortex of the macaque monkey. Proc. R. Soc. Lond. B 213, 73–86 (1981).
Lysakowski, A., Standage, G.P. & Benevento, L.A. An investigation of collateral projections of the dorsal lateral geniculate nucleus and other subcortical structures to cortical areas V1 and V4 in the macaque monkey: a double label retrograde tracer study. Exp. Brain Res. 69, 651–661 (1988).
Bullier, J. & Kennedy, H. Projection of the lateral geniculate nucleus onto cortical area V2 in the macaque monkey. Exp. Brain Res. 53, 168–172 (1983).
Benevento, L.A. & Yoshida, K. The afferent and efferent organization of the lateral geniculo-prestriate pathways in the macaque monkey. J. Comp. Neurol. 203, 455–474 (1981).
Horton, J.C. Cytochrome oxidase patches: a new cytoarchitectonic feature of monkey visual cortex. Phil. Trans. R. Soc. Lond. B 304, 199–253 (1984).
Stepniewska, I., Qi, H.X. & Kaas, J.H. Do superior colliculus projection zones in the inferior pulvinar project to MT in primates? Eur. J. Neurosci. 11, 469–480 (1999).
Yoshida, K. & Benevento, L.A. The projection from the dorsal lateral geniculate nucleus of the thalamus to extrastriate visual association cortex in the macaque monkey. Neurosci. Lett. 22, 103–108 (1981).
Benevento, L.A. & Standage, G.P. Demonstration of lack of dorsal lateral geniculate nucleus input to extrastriate areas MT and Visual 2 in the macaque monkey. Brain Res. 252, 161–166 (1982).
Sorenson, K.M. & Rodman, H.R. A transient geniculo-extrastriate pathway in macaques? Implications for 'blindsight'. Neuroreport 10, 3295–3299 (1999).
Tong, F. Primary visual cortex and visual awareness. Nat. Rev. Neurosci. 4, 219–229 (2003).
Crick, F. & Koch, C. A framework for consciousness. Nat. Neurosci. 6, 119–126 (2003).
Sincich, L.C. & Horton, J.C. Independent projection streams from macaque striate cortex to the second visual area and middle temporal area. J. Neurosci. 23, 5684–5692 (2003).
Shipp, S. & Zeki, S. The organization of connections between areas V5 and V1 in macaque monkey visual cortex. Eur. J. Neurosci. 1, 309–332 (1989).
Yukie, M. & Iwai, E. Laminar origin of direct projection from cortex area V1 to V4 in the rhesus monkey. Brain Res. 346, 383–386 (1985).
Ungerleider, L.G. & Mishkin, M. The striate projection zone in the superior temporal sulcus of Macaca mulatta: location and topographic organization. J. Comp. Neurol. 188, 347–366 (1979).
Hendry, S.H. & Yoshioka, T. A neurochemically distinct third channel in the macaque dorsal lateral geniculate nucleus. Science 264, 575–577 (1994).
Rodman, H.R., Sorenson, K.M., Shim, A.J. & Hexter, D.P. Calbindin immunoreactivity in the geniculo-extrastriate system of the macaque: implications for heterogeneity in the koniocellular pathway and recovery from cortical damage. J. Comp. Neurol. 431, 168–181 (2001).
Malpeli, J.G. & Baker, F.H. The representation of the visual field in the lateral geniculate nucleus of Macaca mulatta. J. Comp. Neurol. 161, 569–594 (1975).
Hendry, S.H. & Reid, R.C. The koniocellular pathway in primate vision. Annu. Rev. Neurosci. 23, 127–153 (2000).
Saito, H., Tanaka, K., Isono, H., Yasuda, M. & Mikami, A. Directionally selective response of cells in the middle temporal area (MT) of the macaque monkey to the movement of equiluminous opponent color stimuli. Exp. Brain Res. 75, 1–14 (1989).
Seidemann, E., Poirson, A.B., Wandell, B.A. & Newsome, W.T. Color signals in area MT of the macaque monkey. Neuron 24, 911–917 (1999).
Callaway, E.M. Local circuits in primary visual cortex of the macaque monkey. Annu. Rev. Neurosci. 21, 47–74 (1998).
Raiguel, S.E., Lagae, L., Gulyas, B. & Orban, G.A. Response latencies of visual cells in macaque areas V1, V2 and V5. Brain Res. 493, 155–159 (1989).
Schmolesky, M.T. et al. Signal timing across the macaque visual system. J. Neurophysiol. 79, 3272–3278 (1998).
Nowak, L.G. & Bullier, J. The timing of information transfer in the visual system. in Cerebral Cortex (eds. K.S. Rockland, J.H. Kaas & A. Peters) 205–241 (Plenum, New York, 1997).
Maunsell, J.H. & Gibson, J.R. Visual response latencies in striate cortex of the macaque monkey. J. Neurophysiol. 68, 1332–1344 (1992).
Nowak, L.G., Munk, M.H., Girard, P. & Bullier, J. Visual latencies in areas V1 and V2 of the macaque monkey. Vis. Neurosci. 12, 371–384 (1995).
Cropper, S.J. & Derrington, A.M. Rapid colour-specific detection of motion in human vision. Nature 379, 72–74 (1996).
Rodman, H.R., Gross, C.G. & Albright, T.D. Afferent basis of visual response properties in area MT of the macaque. I. Effects of striate cortex removal. J. Neurosci. 9, 2033–2050 (1989).
Girard, P., Salin, P.A. & Bullier, J. Response selectivity of neurons in area MT of the macaque monkey during reversible inactivation of area V1. J. Neurophysiol. 67, 1437–1446 (1992).
Barbur, J.L., Watson, J.D., Frackowiak, R.S. & Zeki, S. Conscious visual perception without V1. Brain 116, 1293–1302 (1993).
Collins, C.E., Lyon, D.C. & Kaas, J.H. Responses of neurons in the middle temporal visual area after long-standing lesions of the primary visual cortex in adult new world monkeys. J. Neurosci. 23, 2251–2264 (2003).
Rodman, H.R., Gross, C.G. & Albright, T.D. Afferent basis of visual response properties in area MT of the macaque. II. Effects of superior colliculus removal. J. Neurosci. 10, 1154–1164 (1990).
Stepniewska, I., Ql, H.X. & Kaas, J.H. Projections of the superior colliculus to subdivisions of the inferior pulvinar in New World and Old World monkeys. Vis. Neurosci. 17, 529–549 (2000).
Harting, J.K., Huerta, M.F., Hashikawa, T. & van Lieshout, D.P. Projection of the mammalian superior colliculus upon the dorsal lateral geniculate nucleus: organization of tectogeniculate pathways in nineteen species. J. Comp. Neurol. 304, 275–306 (1991).
Maunsell, J.H., Nealey, T.A. & DePriest, D.D. Magnocellular and parvocellular contributions to responses in the middle temporal visual area (MT) of the macaque monkey. J. Neurosci. 10, 3323–3334 (1990).
Acknowledgements
We thank K.D. Murray for advice on CaMK2 immunochemistry and J. Kaas for comments on the manuscript. The work was supported by The Larry L. Hillblom Foundation and by National Eye Institute grants (to L.C.S., J.C.H.) and the Beckman Vision Center. The California Regional Primate Research Center was supported by a National Institutes of Health Base Grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Tracer injection in area MT did not contaminate cortical white matter. Displayed are the remaining CO-CTB reacted sections from the right hemisphere of Monkey 1, in a continuous series from the section shown in Figure 1 down to white matter. The same location is indicated by the red arrow in all sections. Note that by section 17, only white matter is visible and the injection bolus is no longer present. The possibility of white matter contamination is a significant issue because the optic radiations projecting from the LGN to V1 pass immediately below MT in the intact brain. Scale bar, 1 cm. (JPG 89 kb)
Supplementary Fig. 2
Microphotographs of the LGN sections used for camera lucida plots in Figure 2. At this magnification, only the clusters of WGA-HRP labeled cells are visible, though these sections were reacted for both CTB and WGA-HRP. Scale bar, 1 mm. (JPG 47 kb)
Rights and permissions
About this article
Cite this article
Sincich, L., Park, K., Wohlgemuth, M. et al. Bypassing V1: a direct geniculate input to area MT. Nat Neurosci 7, 1123–1128 (2004). https://doi.org/10.1038/nn1318
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1318
This article is cited by
-
A clinico-anatomical dissection of the magnocellular and parvocellular pathways in a patient with the Riddoch syndrome
Brain Structure and Function (2024)
-
Global motion processing in infants’ visual cortex and the emergence of autism
Communications Biology (2023)
-
Cortical neural dynamics unveil the rhythm of natural visual behavior in marmosets
Communications Biology (2022)
-
Neurophysiological considerations for visual implants
Brain Structure and Function (2022)
-
Conducting Channels in the Visual System. The Third Channel
Neuroscience and Behavioral Physiology (2022)