Article Text
Abstract
Aims: To demonstrate the efficacy of mitomycin C as adjuvant therapy preoperatively and intraoperatively in the management of recurrent or diffuse ocular surface neoplasias.
Methods: The case notes of 11 patients receiving mitomycin C adjuvant therapy as 0.04% eye drops four times a day in two weekly courses preoperatively and/or a single intraoperative application of 0.4 mg/ml of mitomycin C were reviewed. The histopathology included conjunctival primary acquired melanosis, conjunctival melanomas, sebaceous cell carcinomas with conjunctival intraepithelial spread, and conjunctival intraepithelial squamous neoplasias. Seven patients had additional limited local excision of the residual tumour mass and one had cryotherapy.
Results: All cases showed a favourable response to mitomycin C adjuvant therapy with regression in size or retardation of a rapid growth pattern and no serious sequelae. Postoperative follow up of 6–36 months following excision of the lesion with or without intraoperative mitomycin C showed no clinical recurrence in any of the cases.
Conclusion: In this series, mitomycin C adjuvant therapy of recurrent or diffuse ocular surface neoplasias was well tolerated and showed favourable clinical results.
- mitomycin C
- adjuvant therapy
- ocular surface neoplasias
Statistics from Altmetric.com
Topical mitomycin C chemotherapy in the management of ocular surface neoplasias is well documented but it has never gained favour as a primary mode of therapy. This has been mainly the result of poor tolerance and the potential for destructive sequelae. However, its use has been described in conjunctival corneal dysplasias and neoplasias,1–3 conjunctival malignant melanoma and primary acquired melanosis with atypia,4,5 and recurrent conjunctival papillomatosis.6 Other forms of adjuvant therapy for recurrent disease include cryotherapy,7,8 β irradiation,9,10 and immunotherapy.11 We report 11 eyes of 11 patients with a variety of recurrent and/or diffuse ocular surface neoplasias treated with adjuvant topical mitomycin C either as drops or a single intraoperative application.
PATIENTS AND METHODS
The case notes of 11 patients receiving mitomycin C adjuvant therapy as 0.04% eye drops and/or a single intraoperative application of 0.4 mg/ml of mitomycin C between May 1998 and April 2000 were reviewed. A single ophthalmic surgeon at a tertiary referral oncology unit performed the surgical excisions. Mitomycin C chemotherapy was monitored in conjunction with a clinical oncologist.
The study group included two males and nine females, mean age was 66.7 years (range 41–80 years), and mean follow up was 9.5 months (range 1–23 months).
The histopathological diagnosis was conjunctival primary acquired melanosis in three patients, conjunctival melanoma in three patients, sebaceous carcinoma with conjunctival intraepithelial spread in two patients and one each of conjunctival carcinoma in situ, conjunctival squamous carcinoma, and Bowenoid dysplasia.
Adjuvant mitomycin C chemotherapy was indicated for (a) recurrence of lesions following primary excision biopsy, (b) margins not clear on histopathology reports, and/or (c) extensive or diffuse lesions with or without the involvement of the caruncle or canaliculus.
All the cases in the series were referrals from other centres. Six had previous surgical excision at other centres followed by recurrence or the margins were not clear in the biopsy specimens. The remainder were diffuse or extensive lesions. The patients were divided into four groups on the basis of management (Table 1). Group A included four cases of mixed histopathology with discrete lesions who had local excision of the lesions followed by intraoperative application of 0.4 mg/ml of mitomycin for 3–4 minutes to bare sclera. One patient required a conjunctival autograft. Group B included four cases of mixed histopathology with recurrent and extensive lesions who received mitomycin C 0.04% eye drops four times a day for 4 weeks. This was administered as two fortnightly courses separated by 14 days without treatment to allow the limbal stem cells to regenerate. One patient in this group had post-treatment cryotherapy. In the remainder, no further surgical intervention was necessary. Group C included two cases of mixed histopathology who had mass lesions associated with canalicular or caruncular spread. Treatment consisted of preoperative mitomycin C 0.04% drops in the same dosage as group B to reduce the tumour mass followed by limited local excision of the main tumour mass. One patient in this group also had intraoperative application of 0.4 mg/ml of mitomycin C for 3 minutes as the excision was felt to be incomplete at the time of surgery. Group D consisted of a lone case of sebaceous cell carcinoma with extensive intraepithelial spread. The patient had a limited excision of the main tumour mass with intraoperative application of 0.4 mg/ml of mitomycin C for 3 minutes to the bare sclera. A conjunctival autograft was then used to cover the defect. This was followed by a course of topical mitomycin C 0.04% eye drops a few weeks postoperatively. The patient subsequently developed a recurrence that responded to another course of topical 0.04% mitomycin C for 3 weeks.
Treatment groups. (There were four patients in group A, four patients in group B, two in group C and 1 in group D)
RESULTS
All four patients in group A tolerated the intraoperative mitomycin C well and are stable postoperatively with no signs of recurrence of the lesions. In group B two patients showed definite regression of the lesions and developed no side effects of the treatment. One required additional cryotherapy to the residual lesion. The remaining two tolerated the topical mitomycin C drops poorly and treatment was aborted despite signs of regression. In group C, the two patients showed reduction in size of the lesions preoperatively and are stable after excision of the residual lesion. One developed a reaction to the drops during the second fortnightly course of topical mitomycin C drops but treatment was continued under steroid cover. The single patient in group D developed persistent epithelial defects following both courses of topical mitomycin C drops but on both occasions, the epithelial defects responded to lubricants and patching. The patient diagnosed with sebaceous carcinoma with intraepithelial spread was biopsy negative 22 months after her initial mitomycin C treatment. Of the seven patients who received topical mitomycin C 0.04% drops, four developed a reaction to the drops. In two of the four the treatment had to be aborted. None of the patients receiving the intraoperative mitomycin C alone developed any complications to the drug.
Detailed patient data are presented in Table 2 and examples of effects of treatment are shown in Figures 1–3.
Patient data
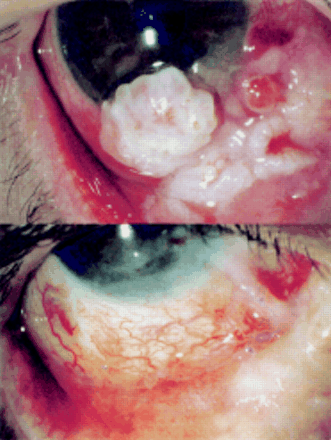
Pretreatment and post-treatment appearance of a recurrent conjunctival malignant melanoma following local excision and intraoperative mitomycin C application. No recurrence of the lesion occurred over 17 months despite margins of the excised lesion not being clear on histopathological examination.
Pretreatment and post-treatment appearance of a recurrent conjunctival malignant melanoma following a single course of mitomycin C 0.04% eye drops. No recurrence of the lesion occurred over 9 months.
Pretreatment and post-treatment appearance of an extensive conjunctival squamous cell carcinoma with canalicular involvement following preoperative mitomycin C 0.04% eye drops, conservative excision of the main tumour nodule, and local intraoperative application of mitomycin C 0.4 mg/ml for 3.5 minutes. Definite regression of the lesion occurred over 6 months and a visual acuity of 6/6 was maintained in the treated eye.
DISCUSSION
Topical and intraoperative local mitomycin C chemotherapy has several advantages when considered in conjunction with limited local excision, especially in recurrent or diffuse lesions. Tumours that present as spreading across tissue planes often dictate the need for radical excision or exenteration that can create significant morbidity. Mitomycin C allows the entire ocular surface including conjunctival fornices to be treated and has a longlasting effect even after cessation of treatment. Adjunctive mitomycin therapy can therefore reduce the level of surgical morbidity.
The use of topical mitomycin C in ophthalmology has been advocated both for its cytotoxic and its antifibrotic activity. Topical use in the form of eye drops has been described for the chemotherapy of conjunctival corneal dysplasias and neoplasias,1–3 primary acquired melanosis and conjunctival malignant melanomas,4,5 and recurrent conjunctival papillomatosis.6 It is known to have an antiproliferative effect on the subconjunctival fibroblasts12 and has been used as an adjunct to the surgical management of pterygium13,14 and glaucoma.15 Its use after excimer laser refractive surgery, as a wound modulator has been reported.16
Mitomycin C is an alkylating antibiotic that acts in all phases of the cell cycle inducing scission of tumour DNA even after treatment has been discontinued, thereby mimicking the effects of ionising radiation.17,18 Rapidly dividing cells are most sensitive. The greatest chance of eradicating all tumour cells is achieved by using the highest possible doses against the smallest possible tumour load.19,20 These properties of mitomycin C support its potential chemotherapeutic effectiveness, as an adjunct to surgical excision. In this study patients in groups A, C, and D underwent surgical excision as a part of their treatment regimens and patients in group B had surgical excision before initiation of their treatment regimen.
Toxicity to topical mitomycin C is widely reported. Wilson et al3 and Demirci et al5 described transient side effects like tearing, photophobia, and punctate epitheliopathy in all patients receiving 0.04% mitomycin C eye drops, but report no serious complications. Ando et al21 concluded that 0.04% mitomycin C was relatively non-toxic to intact corneal epithelium. Intermittent therapy prevents damage to slower growing cells allowing them to repair their DNA and limbal stem cell depletion can be avoided.19,20 In this study the patients in groups B, C, and D received intermittent therapy with mitomycin C in a concentration of 0.04% and no severe complications were seen.
Serious toxicity like scleral ulceration, iridocyclitis, cataract, and glaucoma, has been reported.22–24 These complications were described with the application of mitomycin C to the bare sclera. However, Manning et al,14 in a randomised prospective trial, used intraoperative mitomycin C 0.4 mg/ml applied for 3 minutes following pterygium excision and reported no complications. Rubinfeld et al13 demonstrated the relative safety of 0.2 mg/ml of intraoperative mitomycin C applied for 3 minutes. In this series intraoperative mitomycin C was used in the dose of 0.4 mg/ml for 3–4 minutes and no complications were seen.
Preoperative and intraoperative use of mitomycin C adjuvant therapy made less than radical surgical intervention possible in recurrent and/or diffuse ocular surface neoplasias. A variety of histopathological conditions were treated effectively including the notoriously aggressive intraepithelial sebaceous cell carcinoma. Importantly, there was no need to remove eyes with good vision and the potential for local metastatic spread was curtailed. The proportion of patients in whom treatment was discontinued was small (18.2%). The patients developing persistent epithelial defects had associated secondary cicatricial changes leading to ocular surface abnormalities that may have compounded the toxic response. Both these patients responded to local management with no serious sequelae. This series demonstrates the effective and safe use of mitomycin C chemotherapy as an adjunct to surgical intervention in recurrent and/or diffuse ocular surface neoplasias.