Article Text
Abstract
Aims: To study the clinical properties of double vital staining in premacular fibrosis, facilitating complete removal of all epiretinal tissue.
Methods: In a two step surgery, the epiretinal pucker was removed after staining with trypan blue, whereafter the inner limiting membrane was peeled after staining with infracyanine green.
Results: In all 30 patients, a separate epiretinal layer and inner limiting membrane were removed from the macular area. Pathological examination showed different histological properties of the removed layers. An increased visual acuity was measured in 26 patients, and a slightly decreased visual acuity in one patient.
Conclusion: The described double staining technique could be a novel valuable tool that may help to achieve optimal anatomical and functional recovery after surgery for premacular fibrosis
- vital staining
- infracyanine green
- trypan blue
- epiretinal membranes
- pucker
- inner limiting membrane
Statistics from Altmetric.com
Epiretinal membrane formation in the macular area not infrequently occurs in the population over 50 years of age. The membranes develop as a result of proliferation of cells and deposition of collagen on the retinal surface. The exact cellular origin of the epiretinal tissue is somewhat controversial. Some authors consider the Müller cells as source of the newly formed membranes, while others suggest that fibroblasts from the vascular connective tissue, epithelial cells from the ciliary body, retinal pigment epithelial cells, inflammatory cells, or retinal glial cells are the cells of origin.1
In the initial stage, the ophthalmoscopic appearance is characterised by a mild sheen and hyper-reflectivity in the macular area. In more advanced stages, increased tortuosity of the temporal arcade vessels can be observed, as well as striae and folds in the underlying neural retina. Prolonged traction from the epiretinal membranes can induce macular oedema, macular cyst formation, and even small haemorrhages.
Optical coherence tomography (OCT) can be helpful in assessing the relation between the epiretinal membrane and the underlying neural retina, and in establishing the extent of secondary retinal changes, such as macular oedema or macular cystic degeneration.2 For the latter, fluorescein angiography can also be performed.
Vitrectomy is usually delayed until the more advanced stages, until the epiretinal membrane causes symptomatic visual loss or a disabling metamorphopsia. Epiretinal membranes are sometimes highly transparent resulting in poor visualisation, and often extend more peripherally than suspected from their ophthalmoscopic appearance. Complete removal of all epiretinal tissue can therefore be difficult. Moreover, there is another layer in between the epiretinal tissue and the neural retina that may have a role: the inner limiting membrane (ILM), constituting the innermost layer of the retina. When the epiretinal membrane is of long duration, it creates folding and sometimes tearing of the ILM, which may also contribute to the formation of retinal striae and folds (personal observation). Subsequent ILM removal as an additional step in premacular fibrosis surgery may therefore help to achieve better anatomical and functional results.
It has shown recently that fibrous structures such as proliferative membranes can be stained using trypan blue.3 The ILM can be stained during surgery before removal using indocyanine green4 or infracyanine green.5 Indocyanine green contains iodine to enhance is solubility, and must be dissolved in pure water. Infracyanine green does not contain iodine, precipitates in water, and glucose 5% is rather used as solvent. Since the indocyanine dye is dissolved in 5 ml pure water for injection, and then 1 ml of this solution is diluted in 4 ml BSS Plus, a hypo-osmotic solution is obtained. The addition of indocyanine green molecules minimally alters the osmolarity since it is a large molecule, and is bound to proteins in vivo. As was previously demonstrated, infracyanine green is less likely to induce osmolarity related toxic effects on the retinal pigment epithelial cells.6–8
We performed a study on 30 patients with idiopathic premacular fibrosis using a double staining technique with trypan blue and infracyanine green for separate staining of the epiretinal membrane and the underlying ILM. We tested whether this technique is helpful in delineating and removing both membranes, and whether or not side effects occurred as consequence of this novel technique.
MATERIALS
For vitrectomy and phacoemulsification surgery, a Bausch & Lomb (Rochester, NY, USA) Millennium vitrectomy system was used. When a phacoemulsification was performed, a BL27 (Bausch & Lomb) implant lens was placed within the lens capsule. During the vitrectomy, an EIBOS wide angle viewing system (Möller-Wedel, Wedel, Germany) or a flat high resolution contact lens (DORC, Zuidland, Netherlands, catalogue No 1284DD) were used to obtain optimal viewing. A trypan blue stock solution 0.3% was prepared by dissolving 60 mg trypan blue in 2 ml phosphate buffered saline (PBS) and sterilised by Micropore filtration. A concentration of 0.15% was obtained by further dilution in BSS (Alcon, Forth Worth, TX, USA). Infracyanine green (Laboratoires SERB, Paris, France) was prepared as follows: 25 mg ICG was dissolved in 5 ml of glucose 5% solution, and shaken vigorously. For incising the macular pucker and the ILM, a 19 gauge, non-reflecting sclerotomy knife (Bausch & Lomb) was used. The epiretinal tissue and ILM were grasped with a DORC 1286 W or 1286 B forceps. The removed epiretinal tissue and ILM were immediately fixed in 2.5% glutaraldehyde, 0.1M phosphate buffer. After postfixation in 1% OsO4 0.1M phosphate buffer the specimen was further prepared as for routine electron microscopy. The surgical procedure was recorded using a digital video imaging system from CIT Engineering (Geel, Belgium, http://www.citeng.com).
The intraocular use of trypan blue was approved by the ethics committee of the UZLeuven.
METHODS
In this study, 30 patients were elected with decreased visual acuity secondary to the formation of premacular fibrosis. Preoperative examination included visual acuity measurement, biomicroscopical evaluation, dilated fundus examination, optical coherence tomography of the macular area, and fluorescein angiography in elected patients if other abnormalities were suspected. Informed consent was obtained from all patients. When lens opacities were present and the patient was over 65 years of age, the vitrectomy was preceded by phacoemulsification of the lens and intraocular lens implantation. A three port vitrectomy with vitreous base shaving was performed first, and a fluid/air exchange performed, with removal of most of the intraocular fluid using a blunt back flush needle. A 0.15% solution of trypan blue was then aspirated in a 2 ml syringe, and connected to a blunt tipped needle. With the needle opening close to the retinal surface, approximately 200–500 μl of the solution was sprayed over the macular area in the air filled eye. The air in the vitreous cavity prevented the trypan blue dye from diffusing away from the posterior pole. After 2 minutes, most of the dye was removed with a back flush instrument using the positive air pressure (30 mm Hg), whereafter an air/fluid exchange was performed, to aspirate the remaining dye. The macular epiretinal membrane could then be observed as a light blue stained entity, sharply demarcated from the non-stained neural retina (Fig 1). The stained fibrotic tissue was grasped with an intraocular forceps, and carefully removed from the posterior pole (Fig 2). In the next phase of the surgical procedure, approximately 500 μl of the infracyanine green solution was injected over the posterior pole, while the infusion line was closed. After 2 minutes, the infusion line was reopened, and the excess of the dye removed. The ILM was then incised using a sclerotomy knife, grasped and removed from the posterior pole in a rhexis-like manner (Fig 3).
Intraoperative view of macular pucker before (A) and after (B) staining with trypan blue.
Intraoperative view of removed macular pucker (A), and after consecutive staining with ICG (B).
Removal of the ILM. (A) Incision of the ILM at the upper arcade using a 19 gauge sclerotomy knife. Note the wrinkling of the ILM. (B) Grasping the edge of the incised ILM with a forceps. (C) Wide angle view after removal of the ILM from the macular area. Note small capillary haemorrhages secondary to traction exerted by the ILM on the retina.
RESULTS
In this study, 30 patients were treated for the formation of an idiopathic epiretinal membrane using the above described double staining technique. The average age was 70 years (range 52–80), with equal M/F distribution (16/14). The average follow up time was 23 weeks (range 4–40). Preoperative visual acuities ranged from 20/800 to 20/30 (Fig 4). In this patient group, 25 puckers were idiopathic, four developed after previous vitrectomy for retinal detachment, and one patient underwent a previous buckling procedure for retinal detachment. The vitrectomy was combined with a phacoemulsification procedure and IOL implantation in 17 patients (57%), nine patients (30%) were already pseudophakic before the vitrectomy. In two patients, postoperative lens opacification occurred, requiring cataract surgery. In 14 patients (47%), a gas tamponade with SF6 (15%) was used.
Visual acuity was measured before surgery (axis), and during the last post-operative visit (ordinate). In 26 patients, an increased visual acuity was measured; in three the visual acuity did not change and in one patient a slight decrease in visual acuity was observed. See text for more details.
A complete removal of the visualised epiretinal tissue and underlying ILM was obtained in all patients. Sometimes, small capillary haemorrhages occurred during the removal of the ILM. These haemorrhages disappeared within 24 hours after surgery.
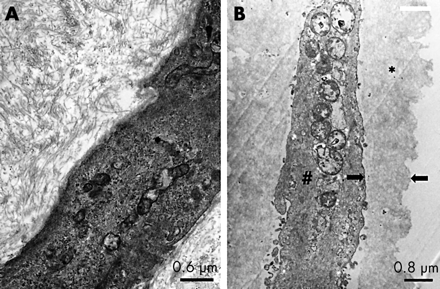
To make certain that the two stained layers corresponded respectively to the epiretinal and internal limiting membrane, both peeled tissue samples were collected in different fixation tubes and examined using electron microscopy (Fig 5). Figure 5A shows a detail of the epiretinal membrane that had been removed after staining with trypan blue. The pucker contained multiple fibrous astrocytes (intermediate type intracellular filaments, interdigitating cytoplasmic processes, and junctional complexes) with myofibroblast differentiation and collagen fibres. The underlying ILM that had been removed after staining with ICG is shown in Figure 5B. The peeled ILM contained scrolled and layered basal membrane material with very few fibrocytes.
Electron microscopic image of removed membranes. (A) Tissue removed after staining with trypan blue. Detail of fibrous astrocyte and collagen fibres. (B) Tissue removed after staining with infracyanine green. Detail of ILM (*) and fibrocyte (#). Arrows indicate the margin of the ILM.
All patients were examined at day 1, 2, 3, and 10 or 14 after surgery. Most patients (60%) reported decreased metamorphopsia in the early postoperative period. In 28 patients (93%), visual acuities returned to preoperative values within 14 days after surgery. In one patient, corneal oedema was still present at day 10, probably secondary to the simultaneously performed phacoemulsification procedure, but disappeared during the following weeks.
In 26 patients (87%), an increased visual acuity was measured during the last postoperative visit (Fig 4). In three patients (10%, two were idiopathic puckers), the visual acuity remained unchanged, but a subjective decreased metamorphopsia was reported. In one patient, who underwent a previous vitrectomy in the same eye for retinal detachment, a decreased visual acuity was measured from 20/100 preoperative to 20/200 postoperative, but there was also reported less distortion of the image in the operated eye.
At present, no adverse effects that could be related to toxic or allergic reactions from the use of these dyes were observed during the follow up period.
DISCUSSION
We have shown that trypan blue and infracyanine green can be used in the same procedure because of the complementarity of the staining properties of both dyes: trypan blue shows affinity for epiretinal membranes as seen in idiopathic premacular fibrosis and less affinity for ILM, as seen in ILM staining during surgery for macular holes (personal observation). In contrast, green dyes are not very useful for epiretinal membranes staining while excellent for ILM visualisation.5,9 This is a surgical alternative for the “negative staining” with indocyanine green.10
It is generally accepted that complete epiretinal tissue removal from the posterior pole is a prerequisite for maximal functional recovery after surgery. Since the epiretinal membrane tissue often extends further than expected by ophthalmoscopy, the described technique using trypan blue staining could be a useful tool to facilitate its complete removal. After epiretinal membrane removal, in most cases the retinal surface still showed visible striae with an overlying folded ILM. Residual tangential traction on the neural retina by folds in the ILM may contribute to the persistence of metamorphopsia and macular oedema that is sometimes observed after macular pucker surgery. Rips in the ILM were also seen after epiretinal membrane removal, probably secondary to prolonged traction exerted by the overlying macular pucker tissue or by adhesion to the removed overlying epiretinal tissue. ICG staining of the ILM was also very useful to facilitate its complete and safe removal.
The safety of the trypan blue staining has been evaluated in a rabbit experimental model injecting trypan blue in a gas filled eye. Despite long term intraocular application, no adverse effects were detected 1 month after injection of the low concentration solution of trypan blue (0.06%). Toxic effects to the retina were only seen after 1 month of exposure to higher concentrations.11 Toxic effects of trypan blue on the retinal pigment epithelial cells were not observed in vitro after a 5 minute contact time even at a concentration of 0.3%.12
The safety and usability of intraocular indocyanine use was previously tested in rabbit experiments,4 in cadaveric eyes,13 and in surgery for macular holes.14 Recent reports have demonstrated a possible toxic effect of indocyanine green on retinal pigment epithelial cells in vitro6 and after surgery for macular holes.7 This toxic effect is probably due to the hypo-osmolarity of the used solvent, and is less likely to occur when infracyanine green dissolved in glucose is used for staining the ILM.8 Therefore, the latter dye was used in this study. Moreover, since during surgery for epiretinal membrane no direct contact between the used dyes and the retinal pigment epithelium is expected, toxic effects are less likely to occur.
During follow up we did not observe any ophthalmoscopic signs of adverse effects of the staining method as used in our patients.
In conclusion, we believe that the described double staining technique could be a novel valuable tool during surgery for premacular fibrosis.
Acknowledgments
Proprietary and financial interest: Dr Gerrit R J Melles has proprietary and financial interest in commercially available solutions of trypan blue for intraocular use (VisionBlue and MembraneBlue, DORC, Zuidland, Netherlands).