Article Text
Abstract
Background Protein tyrosine phosphatases (PTPs) play critical roles in human autoimmunity. Previous studies found that PTPN2 may be the key regulatory factor in the T-cell-mediated immune response. PTPN2 regulates the Janus kinase/signal transducers and activators of transcription pathway by inhibiting signalling via the interleukin (IL)-2 receptor (CD122). An association between genetic variations in PTPN2 and CD122 with ocular Behcet’s disease (BD) has not yet been addressed and was therefore the purpose of this study.
Methods A two-stage case–control study was performed in 906 patients with ocular BD and 2178 healthy controls. Genotyping analysis of 11 single nucleotide polymorphisms was carried out. The expression of PTPN2 in peripheral blood mononuclear cells (PBMCs) was quantified by real-time PCR and cytokine production was measured by ELISA.
Results The frequency of the GG genotype of PTPN2-rs7234029 was significantly lower in patients with ocular BD (p=1.94×10−5, pc=8.34×10−4, OR=0.466). Stratification according to gender showed that rs7234029 was significantly associated with BD in men. A stratified analysis according to the main clinical features showed that rs7234029 was significantly associated with genital ulcers, skin lesions and a positive pathergy test. No association could be detected between BD and CD122 gene polymorphisms. Functional studies showed that rs7234029 GG genotype carriers had a higher PNPT2 mRNA expression level than those which carrying the AA or AG genotype, and a decreased secretion of IL-17 and tumour necrosis factor-alpha was seen by PBMCs from GG carriers. No significant difference could be detected concerning IL-1β or IL-6 production by stimulated PBMCs between the different genotype groups.
Conclusions This study shows that a PTPN2-rs7234029 polymorphism is associated with ocular BD and is strongly influenced by gender. In addition, our results suggest that the genetic association with PTPN2 may involve the regulation of PTPN2 mRNA expression and cytokine secretion.
- genetics
- immunology
- inflammation
Statistics from Altmetric.com
Introduction
Uveitis is a relatively common intraocular inflammatory eye disease which can lead to significant visual impairment. Behcet’s disease (BD) is a common sight-threatening uveitis entity in China1 and is currently considered to be a chronic, intractable autoinflammatory disorder. The main clinical symptoms of BD include a recurrent uveitis, recurrent oral ulceration, genital ulceration and skin lesions, whereby systemic vasculitis was recognised as the main pathological feature. However, musculoskeletal, neurological and gastrointestinal systems can also be affected. Cases of BD have been reported worldwide, but the prevalence of the disease is much higher in countries along the ancient ‘Silk Road’, from Mediterranean to the Far East, such as China, Japan and Turkey.2
The eye is the most commonly involved organ and ocular involvement belongs to the main disabling features of BD. The exact cause of BD remains unknown, but recent studies suggest that both genetic and environmental factors are involved. Two recent large genome-wide association studies (GWAS) reported an association of single nucleotide polymorphisms (SNPs) of interleukin (IL)-10 and IL-23R/IL-12RB2 genes with BD.3 4 Other studies showed that a large number of other genetic factors were also involved in the susceptibility to BD, such as HLA-B51.5 The study of the involvement of genetic factors not only enlarges our knowledge in the pathogenesis of BD, but also allows the development of novel therapeutic tools to prevent the sight-threatening consequences of this disease.
Recently attention has been drawn to the role of protein tyrosine phosphatases (PTPs) gene polymorphisms in the pathogenesis of several human autoimmune diseases, although little is known concerning their involvement with BD.6 They are subdivided into receptor-type PTPs (CD45 and CD148) and non-receptor-type PTPs (PTPN22, SHP-1, PTPN2 and PTP-PEST).7 PTPN22, located on chromosome 1p13, is an important negative regulator of T-lymphocyte function, and PTPN22 variants have been found to be correlated with various immune-related disorders.8 In a previous study from our group, we were however unable to show an association between PTPN22 variants (rs2488457, rs1310182 and rs3789604) with ocular BD in Chinese Han.9 PTPN2, which is located on chromosome 18p11, is a key regulatory factor in the T-cell-mediated immune response.10 Its action is mediated via a negative regulation of the Janus kinase/signal transducers and activators of transcription (JAK/STAT) pathway following signalling via the IL-2 receptor (CD122).10 The JAK1/STAT3 pathway is activated in BD, possibly through elevated expression of type 1 T helper (Th1)/type 17 T helper (Th17)-type cytokines such as IL-2.11 Recently, the PTPN2 variant rs1893217 was found to be associated with the risk of BD development in a Han Chinese population.12 In this latter study, only 28.5% of the patients had uveitis and we therefore decided to expand this study and to focus on a group of patients with BD with ocular involvement. In view of the known interaction between PTPN2 and CD122 signalling pathways, we also included an analysis of CD122 gene variants in our study. No association was found with CD122 but a polymorphism of PTPN2 rs7234029 was shown to affect predisposition to ocular BD.
Materials and methods
Study population
For this project, 906 patients with BD were recruited. Both the patients and healthy controls were recruited at the First Affiliated Hospital of Chongqing Medical University (Chongqing, China) from May 2008 to July 2015. All the enrolled patients with BD and healthy controls were Han Chinese. All the patients had uveitis, whereby most had panuveitis (95.25%), and only 43 patients (4.75%) had anterior uveitis. Patients with BD were strictly diagnosed according to the criteria of the International Study Group for Behcet’s Disease.13 The normal control group comprised 2178 unrelated normal Chinese Han volunteers with no history of any eye disorder or autoimmune disease, and originated from the same regions as the BD group. There was no significant difference in age distribution between the BD and control groups. All the investigated subjects provided their informed written consent to participate in this study before enrolment. The procedures of this study were conformed the ethical guidelines of the Declaration of Helsinki.
SNP selection
Based on the results of available literature reports,12 14–20 we selected the SNPs which were earlier shown to be associated with a variety of autoimmune or autoinflammatory diseases. SNPs with a minor allele frequency of <5% in the Chinese Han population were excluded. Linkage disequilibrium was tested using Haploview software V.4.2. Finally, we selected five SNPs (rs2542151, rs1893217, rs657555, rs478582 and rs7234029) for PTPN2 and six SNPs for CD122 (rs743777, rs9622555, rs743776, rs228941, rs3218253 and rs2284033).
DNA extraction and genotyping
We extracted genomic DNA with the QIAamp DNA Blood Mini Kit (Qiagen) from peripheral blood samples of all the subjects. The SNP genotyping assay was performed by the Mass ARRAY System (Sequenom, San Diego, California, USA) in strict accordance with standard procedures.
Cell isolation and culture
PBMCs were obtained from venous blood samples of 40 healthy genotyped controls using Ficoll-Hypaque density-gradient centrifugation, seeded in 24-well plates (1×106 cells/well) and cultured in Roswell Park Memorial Institute (RPMI) 1640 medium including 100 U/mL penicillin, 10% FCS and 100 µg/mL streptomycin. PBMCs were treated with anti-CD3 antibody (5 µg/mL; eBioscience) and anti-CD28 antibody (1 µg/mL; eBioscience) (5:1) to simulate antigen presentation at 37°C for 72 hours.
Real-Time PCR
To examine the expression of the PTPN2 gene, we extracted total RNA from PBMCs using a commercial reagent (TRIzol; Life Technologies), and reverse transcription was performed using a commercial kit (ABI). Real-time quantitative PCR was carried out by a 7500 real-time instrument (ABI). The expression of PTPN2 was detected using the following primers (PTPN2, forward, 5′-CGGGAGTTCGAAGAGTTGGATA-3′; reverse, 5′-CGACTGTGATCATATGGGCTTA-3′) based on the SYBR-Green method. The primers of β-actin were as below: forward, 5′-GGATGCAGAAGGAGATCACTG-3′; reverse, 5′-CGATCCACACGGAGTACTT-3′. Relative expression levels of PTPN2 were quantified by the 2−ΔΔCt method.
Measurement of cytokines by ELISA
Cytokine levels (tumour necrosis factor-alpha (TNF-α), IL-17, IL-1β and IL-6) in supernatants of PBMCs were quantified using Duoset ELISA development kits (R&D Systems).
Statistical methods
The Hardy-Weinberg equilibrium (HWE) of all tested SNP was determined by the χ2 test. Genotype distributions and allele frequencies of candidate SNPs were evaluated using the χ2 test with SPSS (V.17.0). The Bonferroni correction method was used for correcting the p values for multiple comparisons. The independent samples test or non-parametric Mann-Whitney U test was used to compare PTPN2 expression levels and cytokine levels (IL-1β, IL-6, IL-17 and TNF-α) among different genotype groups.
Results
The clinical characteristics of the enrolled patients with BD and healthy controls are shown in table 1. All the patients with BD had uveitis and attended our uveitis clinic for their eye problems.
Clinical characteristics of patients with BD included in the current study
Genotype and allele frequencies of tested SNPs in the first stage study
In the first phase, 11 SNPs were genotyped in 375 patients with BD and 598 healthy controls. The AG genotype of rs7234029 was significantly associated with BD (pc=2.16×10−2, OR=1.59) (table 2). The GG genotype of rs7234029 in BD patients showed a protective effect as compared with healthy controls (pc=4.43×10−2, OR=0.42) (table 2). No significant association was observed in the other 10 SNPs between ocular BD and healthy controls.
Genotype and allele frequencies of PTPN2 and CD122 genes in ocular BD
Genotype and allele frequencies of SNPs in the replication phase and combined study
To validate the detected association of PTPN2 with BD that was observed in the first phase study, we recruited a separate set of 531 patients with BD and 1580 normal controls for a replication phase. In this replication study, we confined ourselves to the SNP rs7234029, which revealed a statistically significant result in the first phase. The AG genotype of rs7234029 again showed a significant susceptibility to ocular BD (p=1.08×10−5, pc=4.64×10−4, OR=1.556, 95% CI 1.227 to 1.896), and the frequency of the GG genotype of rs7234029 in patients with BD was again significantly lower than that seen in the healthy controls (p=1.24×10−3, pc=5.33×10−2, OR=0.451, 95% CI 0.275 to 0.740) (table 2). Combination of the data from the first and replication study also showed that rs7234029 was significantly associated with BD (AG genotype, p=3.33×10−8, pc=1.43×10−6, OR=1.549; AA genotype, p=1.10×10−3, pc=4.73× 10−2, OR=0.772; for GG genotype, p=1.94×10−5, pc=8.34×10−4, OR=0.466).
Stratified analysis according to gender showed that rs7234029 was significantly associated with BD in men (AG genotype: p=9.32×10−6, pc=4.01×10−4, OR=1.51; GG genotype: p=1.66×10−5, pc=7.14×10-4, OR=0.408) but not in women (online supplementary table 1). Univariate and multivariate logistic regression analyses were also performed adjusting for age and gender. Logistic regression analysis showed that rs7234029 was associated with BD in a co-dominant model, dominant model, recessive model and overdominant model (online supplementary table 2). In the co-dominant model, compared with patients carrying the AA genotype, patients carrying the AG genotype have an increased risk (OR=1.38, p=2.41×10−4), while patients carrying the GG genotype have a decreased risk (OR=0.52, p=8.88×10−4). In the dominant model, patients carrying the AG or GG genotypes have an increased risk (OR=1.23, p=1.58×10−2) compared with those carrying the AA genotype. In the recessive model, patients carrying the GG genotype have a decreased risk (OR=0.44, p=2.23×10−5) compared with those carrying AA or AG genotypes. In the overdominant model, patients carrying the AG genotype have an increased risk (OR=1.49, p=3.18×10−6) compared with those carrying the AA or GG genotypes. We performed a stratified analysis to examine the association of rs7234029 with the main clinical features of BD. The result showed that rs7234029 was significantly associated in patients with BD with a positive pathergy test and with those having skin lesions and genital ulcers. No significant association was found for the other extraocular manifestations of BD with rs7234029 (table 3).
Supplementary file 1
Genotype and allele frequencies of rs7234029 in patients and controls stratified by the main clinical features
The influence of rs7234029 on the PTPN2 expression and cytokine secretion
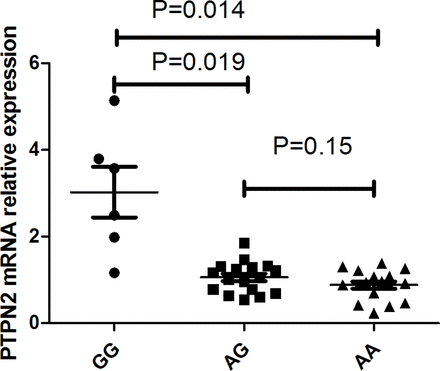
Functional experiments were performed to determine whether PTPN2 mRNA expression was influenced by the different genotypes of rs7234029. Our results revealed that individuals with the AA and AG genotype had a significantly decreased PTPN2 mRNA expression when compared with GG individuals (p=0.014 and p=0.019) (figure 1). We further determined whether different genotypes of rs7234029 influenced PBMC cytokine secretion in healthy individuals. An elevated secretion of IL-17 (p=0.018) and TNF-α (p=0.034) was detected in GG genotype as compared with AA genotype individuals. No significant difference could be detected concerning IL-1β or IL-6 production by stimulated PBMCs in different genotype individuals (figure 2).
The influence of rs7234029 on the expression of PTPN2 mRNA. The expression of PTPN2 mRNA in PBMCs treated with anti-CD3/28 antibodies. PBMCs were obtained from healthy individuals with diverse genotypes of rs7234029 (GG=6, AG=17, AA=17). Data show the mean ±SD. PBMCs, peripheral blood mononuclear cells; PTP, protein tyrosine phosphatase.
The influence of rs7234029 on the cytokine production. The production of IL-17, TNF-α, IL-1β, IL-6 in PBMCs treated with anti-CD3/28 antibodies from normal controls carrying different genotypes of rs7234029 (GG=6, AG=16, AA=16). IL, interleukin; PBMCs, peripheral blood mononuclear cell; TNF-α, tumour necrosis factor-alpha.
Discussion
We investigated the association of 11 SNPs of PTPN2 and CD122 with BD in a Chinese Han population. The results revealed that the AG genotype of PTPN2 rs7234029 conferred a disease risk for BD, whereas the GG genotype of this locus had a protective effect on BD. Functional studies showed GG genotype of rs7234029 carriers had a higher PNPT2 mRNA expression level than those carrying the AA or AG genotype. Additionally, the secretion of TNF-α by PBMCs was significantly lower in rs7234029 GG genotype cases as compared with the AA or AG genotype cases. Secretion of IL-17 by PBMCs was significantly lower in individuals with the GG genotype of rs7234029 than those with the AG genotype. The protective GG genotype is thus associated with an intrinsic lower capacity to respond with proinflammatory cytokines following an antigenic stimulus.
PTPN2 is a non-receptor tyrosine-protein phosphatase which is a member of the PTP family. It encodes the T-cell PTP (TC-PTP) that shows a ubiquitously high expression in haematopoietic cells. PTPN2 is involved in the IL-2 receptor-activated JAK-STAT signalling pathways as a negative regulator, whereby PTPN2 negatively regulates T-cell activation by inhibiting IL-2 receptor signalling.21 22 It has been shown that PTPN2 plays a crucial role in the pathogenesis of chronic intestinal inflammation and dysfunction of PTPN2 was considered to play an important role in the development of autoimmunity.23 This latter study showed that loss of PTPN2 causes increased levels of Th1 and Th17 cells but reduced levels of regulatory T cells (Tregs), and that the CD-associated PTPN2 variant led to increased Th1-associated and Th17-associated gene expression in intestinal samples from patients with inflammatory bowel disease (IBD).23 Others have demonstrated that signalling of CD4 +T cells via the IL-2Rβ chain is affected by variants of PTPN2 rs1893217.10
GWAS studies have identified PTPN2 as a susceptibility gene for IBD. The Welcome Trust Case Control Consortium GWAS studies demonstrated an associations between Crohn’s disease and variants in PTPN2 rs7234029 in a British population (p=3.71×10–7).24 A Caucasian study also showed that PTPN2 SNP rs7234029 (G allele) was associated with CD (p=1.30×10–3, OR 1.35, 95% CI 1.13 to 1.62) and provided evidence showing that rs7234029 modulates the binding sites of transcription factors involved in inflammation.16 In another study, rs7234029 was also observed to be associated with Crohn’s disease.25 Similar outcomes were recently found in European American individuals with juvenile idiopathic arthritis (JIA) (rs7234029, p=7.19×10–11, OR=1.59; rs1893217, p=3.48×10–8, OR=1.52; rs2542151, p=3.05×10–7, OR=1.45).26 Our results confirm the previous study in which PTPN2 rs7234029 was shown to be associated with BD. We were not able to reproduce earlier findings from a Chinese group who found that PTPN2 SNP rs1893217 showed a weak association with the risk of BD in a Han Chinese population (pc=0.04).12 They also tested PTPN2 rs7234029 but did not find a significant association in their BD group. The discrepancy may be caused by the different clinical features of the patients included in the two studies. In their study,12 the patients were all recruited at the Department of Rheumatology and Clinical Immunology and only 28.5% of patients exhibited ocular manifestations, whereas all the enrolled patients in our study had uveitis. There was no stratified analysis according to the main clinical features in the latter study, which means that it is not possible to determine the relationship between rs1893217 and ocular BD in that population. However, all the enrolled patients had uveitis in our study. Our results showed that a PTPN2-rs7234029 polymorphism is associated with ocular BD but is not associated with hypopyon. This might be due to the fact that only 223 patients (24.6%) had hypopyon in our study and that the sample size might have been too small to obtain statistical significance. Uveitis in patients with BD usually presents with a transient hypopyon in 25% of cases in our clinical practice. Further studies are needed to investigate the association between different uveitis disease manifestations and the tested gene polymorphisms.
In our study, we found that PTPN2 SNP rs7234029 was significantly associated with BD (AG genotype, p=3.33×10−8, pc=1.43× 10−6, OR=1.549; AA genotype, p=1.10×10−3, pc=4.73×10−2, OR=0.772; for GG genotype, p=1.94×10−5, pc=8.34×10−4, OR=0.466). Further analysis showed that rs7234029 was significantly associated with BD in men but not in women, although the trend was the same. Rs7234029 was significantly associated in patients with BD with genital ulcers, skin lesions and a positive pathergy test. This might seem discrepant since genital lesions for instance were observed more frequently in women than in men (64.2% vs 54.8%, p=0.033), whereas the frequency of skin lesions was similar between men and women (77.1% vs 70.2%, p>0.05) in our study. All the patients in our study had uveitis and men usually showed a more severe sight-threatening uveitis than women.27 This combined with the fact that men were often referred to us preferentially might have resulted in a male patient bias in our study. The six SNPs of CD122 were not associated with BD in our study, although previous studies did show an association with autoimmune diseases. The disparity between the results may be due to the different genetic backgrounds for BD and the other autoimmune diseases.
PTPN2 variants have been demonstrated to be associated with an impairment of IL-2R signalling in CD4 +T cells,10 and the rs7234029 variant may affect activation of proinflammatory transcription factors.10 The JAK1/STAT3 signalling pathway is activated in BD, leading to an elevated expression of Th1/Th17-type cytokines.11 Th1/Th17-type cytokines have been implicated in the pathogenesis of many autoimmune diseases. We performed functional assays to investigate whether SNP rs7234029 influenced gene expression and the results showed that the PTPN2 mRNA expression in rs7234029 AA and AG carriers was significantly decreased compared with those carrying the GG genotype. Further investigations found that a decreased secretion of IL-17 and TNF-α was seen by PBMCs from GG carriers. This suggests that more PTPN2 inhibits these cytokines. This is not completely supported by our data, since AG individuals produced significantly more IL-17 than GG individuals while AA individuals did not. We do not yet have an exact answer for this discrepancy. Phenotype–genotype interactions may be involved in the JAK1/STAT3 signalling pathway, and further research needs to be done to elucidate the exact mechanisms involved. Our results are in accordance with a recent study which showed that inflammation-induced interferon (IFN)-γ, TNF-α, IL-17 and IL-6 serum levels were elevated in mice harbouring PTPN2-deficient T cells, and with data showing that a loss-of-function PTPN2 variant led to increased serum levels of IFN-γ and IL-17 in patients with BD.23
Our study suffers from several limitations. First, our participants were all Chinese Han and the patients with ocular BD all came from a Department of Ophthalmology. Further studies should also enrol patients with BD without uveitis coming from other clinical departments and compare the PTPN2 disease association in patients with BD expressing various clinical features of the disease. Second, 83% of our patients with BD were men, while 56% of controls were men. Stratified analysis according to gender showed that rs7234029 was significantly associated with BD in men but not in women. This might be due to the fact that the sample size of our female patient group was too low. Our research was limited to uveitis in BD and should not be extrapolated to other uveitis entities. Further research is also needed to investigate the exact role of the IL-2 receptor-activated JAK-STAT signalling pathways in BD uveitis.
In conclusion, our results revealed that PTPN2 rs7234029 polymorphisms were associated with BD and were strongly influenced by gender. In addition, we provide evidence which suggests that the association with PTPN2 variants may be explained via a regulation of PTPN2 mRNA expression and cytokine secretion.
Acknowledgments
The authors would like to thank all donors enrolled for the present study. They thank Gangxiang Yuan, Qingfeng Cao and Guo Huang for collecting the clinical data.
References
Footnotes
QZ and HL contributed equally.
Contributors PY, QZ and HL: take responsibility for the integrity of the data and the accuracy of the data analysis. QZ and HL: study concept and design; drafting of the manuscript. All authors: acquisition, analysis or interpretation of data; critical revision of the manuscript for important intellectual content. SH, HY and GS: statistical analysis.
Funding This work was supported by National Key R&D Program of China (grant no. 2016YFC0904000), Natural Science Foundation Major International (Regional) Joint Research Project (81320108009), National Natural Science Foundation Project (81300754, 81400389, 31370893), Chongqing Key Laboratory of Ophthalmology (CSTC, 2008CA5003), National Key Clinical Specialties Construction Program of China, Chongqing Science & Technology Platform and Base Construction Program (cstc2014pt-sy10002), Research fund for Traditional Chinese Medicine of Chongqing Health and Family Planning Commission (ZY201401013), Project of Health Bureau of Chongqing (2016MSXM003) and Chongqing applied basic research projects and cutting-edge technology (cstc2014jcyjA10111).
Competing interests None declared.
Patient consent Obtained.
Ethics approval The protocol was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University, Chongqing, China (Permit Number: 2009-201008).
Provenance and peer review Not commissioned; externally peer reviewed.
Linked Articles
- At a glance