Article Text
Abstract
Background Corneal transplant failure with neovascularisation is a leading indication for full-thickness grafts in patients. Lymphangiogenesis is implicated in the pathology of graft failure, and here we systematically evaluate failed human corneal transplants with neovascularisation for the presence of lymphatic vessels.
Methods Nine failed grafts with neovascularisation, based on H&E staining with subsequent immunoperoxidase staining for CD31, a blood vessel marker, were selected. Lymphatics were investigated by immunohistochemical and immunofluorescence approaches using podoplanin as a lymphatic marker. In two of nine cases, fluorescence in situ hybridisation (FISH) was used for detection of lymphatic mRNAs including podoplanin, VEGFR-3 and LYVE-1. All immunofluorescence and FISH samples were compared with positive and negative controls and visualised by confocal microscopy.
Results Corneal neovascularisation was established in all cases by H&E and further confirmed by CD31 immunoreactive profiles. Immunohistochemistry for the podoplanin antibody was positive in all cases and showed morphologies ranging from distinct luminal structures to elongated profiles. Simultaneous immunofluorescence using CD31 and podoplanin showed lymphatic vessels distinct from blood vessels. Podoplanin immunofluorescence was noted in seven of nine cases and revealed clear lumina of varying sizes, in addition to lumen-like and elongated profiles. The presence of lymphatic mRNA was confirmed by FISH studies using a combination of at least two of podoplanin, VEGFR-3 and LYVE-1 mRNAs.
Conclusions The consistent finding of lymphatic vessels in failed grafts with neovascularisation implicates them in the pathogenesis of corneal transplant failure, and points to the lymphatics as a potential new therapeutic target.
- cornea
- experimental – laboratory
- neovascularisation
- pathology
- anatomy
- lymphatic
- vessels
- lymphangiogenesis
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- cornea
- experimental – laboratory
- neovascularisation
- pathology
- anatomy
- lymphatic
- vessels
- lymphangiogenesis
Introduction
Corneal transplantation is a widespread procedure, with 185 000 corneal transplantations performed in 2012.1 Nevertheless, due to the shortage of donor graft tissue, only 1 in 70 patients requiring a corneal transplant receives the surgery.1 Additionally, a recent study encompassing 1132 patients found that, while controlling for confounding prognostic variables, corneal graft survival has not changed in the past 30 years.2 Due to the global demand for corneal transplantation and limited supply of graft tissue, it is important to keep transplanted corneas viable and prevent graft failures. The cornea physiologically lacks blood vessels, which is necessary to maintain the transparency required for vision.3 This avascularity helps ensure the relatively high success of corneal transplantations by hindering immune-mediated rejection of corneal grafts.3 Despite the cornea’s ‘immune privileged’ status, graft rejection may still occur.3
Immune-mediated corneal graft rejection is a major cause of graft failure. Corneal neovascularisation is thought to be an important host factor predisposing transplanted corneas to graft rejection.4 Growth of new blood vessels has long been acknowledged to have a significant role in corneal graft rejection. However, the role of lymphatic vessels remains less well understood, in part due to the lack of known lymphatic vessel markers until more recently.3 A mouse study by Dietrich et al demonstrated that the presence of lymphatic vessels in the grafted tissue had a more significant detrimental effect on graft survival as compared with the presence of blood vessels.5 Several studies to date have investigated the role of lymphatics in various corneal pathologies. One recent paper by Narumi et al on human corneas examined the role of dendritic cells and their relationship to corneal lymphatic and blood vessels in cases with corneal infection.6 Additional studies investigated lymphatics in human corneal samples with varying underlying pathologies including abnormal vessel growth.7–9 However, these studies did not focus specifically on the role of lymphatics in corneal graft failure with neovascularisation.
A 10-year study from 2004 at Queen Victoria Hospital in the UK found graft failure to be the leading indication for penetrating keratoplasty.10 Another study, examining statistics from 2002 to 2012, found graft failure to be the most prevalent indication for penetrating keratoplasty at the University of Washington and University of California, Davis in the USA.11 A recent 25-year study, by the University of Auckland, New Zealand, found graft failure to be the second most common indication for any type of corneal transplant.12 We recently conducted two studies spanning five total years from 2012 to 2016 at the University of Toronto13 ,14 and found that failed transplant was the leading indication for full-thickness graft.13 14 To date, there are very few studies that have investigated lymphatics in human graft failure specimens.9 15 Here, we systematically investigate lymphatics in failed grafts with neovascularisation using multiple approaches.
Materials and methods
Tissue collection
Cases were selected from failed corneal transplants in the University of Toronto Ophthalmic Pathology database from 2013 to 2016. There was a total of 273 failed transplant cases. Thirty-nine of these cases contained documented neovascularisation. Of these, nine cases were found to also contain suspected lymphatics due to podoplanin positivity seen on immunohistochemistry (IHC). After institutional Research Ethics Board approval, these nine cases (six males, three females) of failed corneal transplants with neovascularisation were selected for further study. Six control cases (three males, three females) with healthy corneas were chosen, from which conjunctival tissue was obtained for positive controls. These control cases were acquired from the Human Eye Biobank for Research and ranged in age from 22 to 67. The average age of patients with failed transplant was 65.1±20.1 years (mean±SD). Eight of these cases were failed penetrating keratoplasty grafts and one was failed Descemet’s stripping automated endothelial keratoplasty graft. Based on our findings (see the Results section), two cases were selected for secondary experiments using fluorescence in situ hybridisation (FISH) in order to expand on our findings from the immunohistochemical and immunofluorescent analyses. This is discussed in further detail in the Results section.
Tissue processing
Tissues were immersion fixed in 10% neutral buffered formalin and routinely processed. Paraffin-embedded tissue blocks were sectioned sagittally (8 µm). Sections were mounted onto adhesive microscope slides (TruBond 380, Newcomer Supply, Wisconsin, USA) for further investigation.
Histological staining and bright field microscopy
H&E staining was performed using a standard procedure with a Leica autostainer (ST5010 Autostainer XL, Leica Biosystems, Ontario, Canada). Immunoperoxidase staining of podoplanin (D2-40; Cedarlane Laboratories, Ontario, Canada) was performed as it has been described previously.16 D2-40, a marker of lymphatic vessel endothelium,17 is an antibody that is designed to target a podoplanin protein epitope modified after formalin fixation. Sections were viewed and imaged with an upright microscope (BX51, Olympus, Tokyo, Japan) equipped with a CCD camera (Optronics, California, USA).
Immunofluorescence (IF) staining
Sections were deparaffinised and rehydrated using the Leica Autostainer XL. Antigen retrieval was performed by heating slides in citrate buffer (10 mM sodium citrate, 0.05% Tween 20, pH 6.0) using a Biocare Medical Decloaking Chamber (Biocare Medical, LLC, Concord, California, USA). Sections were washed three times in 1× phosphate-buffered saline (PBS, pH 7.4) for 5 min each, followed by incubation in blocking solution for 1 hour with 2% goat serum (Sigma-Aldrich, Missouri, USA) and 0.3% Triton X-100 (Sigma-Aldrich, Missouri, USA) in 1× PBS. Sections were then incubated in blocking solution with primary antibodies D2-40 (1:100, mouse monoclonal, Cedarlane Laboratories) and CD31 (1:20, rabbit polyclonal, Abcam, Cambridge, UK) overnight at 4°C. CD31 was used as a marker of blood vessel endothelial cells.18 After three 10 min 1× PBS washes, sections were incubated with goat antimouse Alexa Fluor-555 (1:100; Thermo Fisher Scientific, Ontario, Canada) and goat antirabbit Alexa Fluor-647 (1:100; Thermo Fisher Scientific) secondary antibodies in blocking solution for 1 hour in the dark. After three washes in 1× PBS for 10 min each, the sections were covered with a coverslip (#1.5, Thermo Fisher Scientific) and aqueous mounting medium (Dako, Agilent Technologies, California, USA). All sections were treated at room temperature with mild agitation unless otherwise noted.
FISH for lymphatic mRNA
FISH was performed using RNAscope multiplex assay kit and probes (ACDbio; Bio-Techne, Minneapolis, Minnesota, USA). Cornea sections were treated as recommended by the manufacturer. In brief, deparaffinised sections were pretreated for antigen retrieval and protein digestion. After that, they were hybridised with probes specific for three different markers of lymphatic endothelial cells: podoplanin, VEGFR-3, or LYVE-1 18 along with positive or negative controls. POLR2A, cyclophilin B and ubiquitin C probes were used as positive controls. A probe recognising a bacterial antigen was used as a negative control. This procedure was followed by signal amplification steps, 4′,6-diamidino-2-phenylindole (DAPI) staining and mounting in ProLong Gold mounting medium (Invitrogen, USA). The amplification steps involve binding of two independent probes to an RNA sequence in tandem (‘double Z’ probes). A single independent probe will not bind to preamplifier molecules without an adjacent probe bound to the RNA in tandem, thus increasing the specificity of this method. Once these probes are bound to target RNA in tandem, preamplifier molecules bind to a distinct area formed by bound ‘double Z probes’. Next, amplifier molecules bind to binding sites attached to each preamplifier. Fluorescent label probes are then added and adhere to the binding sites on each amplifier. After these steps, a fluorescent signal can be observed corresponding to the target RNA (Advanced Cell Diagnostics, 2015)
Confocal microscopy
Both IF and FISH images were acquired using a Zeiss confocal laser-scanning microscope (LSM 700, Carl Zeiss Microscopy GmbH, Jena, Germany) at 10× and 20× magnification as well as 63× magnification with oil immersion. Multiple images were acquired for each sample using the DAPI, Alexa Fluor-488, Alexa Fluor-555 and Alexa Fluor-647 channels of the Zen Black programme. All images of pathological and non-pathological control corneas were captured under similar conditions and analysed manually using image analysis software (ImageJ, NIH, Bethesda, Maryland, USA). This analysis involved assessing for the presence and measuring the size of potential lymphatics or blood vessels. Based on appearance, podoplanin-positive profiles were classified as ‘lumen’, ‘lumen-like’ or ‘elongated’. The ‘lumen’ category demonstrated a complete lumen, ‘lumen-like’ demonstrated at least 70% of the circumference of a lumen and often had a less distinct morphology than those of the ‘lumen’ category. ‘Elongated’ refers to elongated immunoreactive profiles.
Results
Podoplanin-immunoreactive lymphatics were detected in all nine failed grafts by IHC, seven of which were positive by IF. Two of the nine cases that were evaluated for lymphatic markers via mRNA FISH were found to be positive for at least two lymphatic markers simultaneously. Table 1 summarises staining and demographic data for each case.
Demographic data for failed grafts
Table 1 provides demographic and clinical data as well as a summary of all cases that were positive for lymphatic markers using the various indicated methods. The case ID numbers used coincide with those used in our figures. Age, gender and failed graft type for each case is documented. Presence of neovascularisation for each case is indicated. Presence or absence of podoplanin-positive lymphatics in each case via IHC or IF is denoted. Presence or absence of one or more positive markers used in FISH (podoplanin, VEGFR-3, LYVE-1) is also indicated. Cases where FISH analysis was not performed are designated as ‘N/A’.
Not all cases were positive for lymphatics using both IF and immunoperoxidase (IHC). After stratifying our cases into IHC+/IF+ and IHC+/IF− groups, FISH was performed for each of these scenarios in order to expand on IF and IHC findings. H&E stained sections of failed grafts showed mononuclear inflammatory cells at both low (figure 1A) and high (figure 1B) power. Also noted on H&E were luminal blood vessels (figure 1B—arrows). All grafts were found to be positive for podoplanin on IHC (figure 1C,D—arrows). Neovascularisation was confirmed in every case of corneal graft failure by detection of CD31-positive profiles (figure 2A,C).
Neovascularisation and suspected lymphatics within failed corneal grafts. (A, B and D) Case ID #1: an 88 year-old male patient with failed graft. (C) Case ID #2: an 87-year-old female patient with failed graft. All nine cases of corneal graft failure were found to contain neovascularisation, which is demonstrated above on H&E (A and B). Blood vessels are outlined by a rectangular area shown at 20× magnification (A). Blood vessels are indicated by arrows at 40× magnification (B). Monocellular infiltrates were seen around the lumen of these vessels. All nine cases of corneal graft failure were found to contain luminal and elongated profiles via podoplanin antibody (D2-40) immunohistochemistry. Two immunoperoxidase images are provided above with podoplanin-antibody staining lymphatics denoted by arrows (C and D). Scale bars represent (A) 100 µm and (B–D) 50 µm.
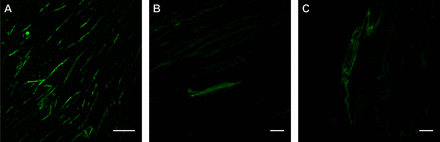
Discrete lymphatics in failed corneal grafts. (A–C) Case ID #2: an 87-year-old female patient with failed graft. (A) Blood (red) and (B) lymphatic vessels (green) are seen in immunofluorescence images in separate CD31 and podoplanin channels, respectively, as well as a merged image (C) at 20× magnification. These vessels are seen as discrete structures and do not colocalise (C). All scale bars for this figure represent 50 µm.
CD31 immunoreactive blood vessels were observed in all cases (figure 2A), yet were distinct from podoplanin antibody immunoreactive lymphatic vessels (figure 2B,C). Negative controls did not show any fluorescence compared to podoplanin and CD31 staining (online supplementary figure 1). Varying lymphatic sizes and morphologies were seen both among separate cases and within a single case (figures 3 and 4, respectively). For example, lymphatics in figure 3 ranged in size from 10 to 54 µm and varied in morphology from narrow, occasionally conjoined, oval profiles to more open, irregular spherical profiles. The figure 4 demonstrated lymphatics ranging in size from 9 to 84 µm with varied morphologies including circular, elliptical and sharp-edged profiles. The figure 5 provides additional examples of the spectrum of unique lymphatic morphologies seen and includes ‘elongated’ (figure 5A,B) and ‘lumen-like’ profiles (figure 5C). The lymphatic sizes in figure 5 ranged from 35 to 39 µm and included areas with multiple (figure 5A) or more scarce (figure 5B) elongated profiles as well as a lumen-like profile with an irregular oval morphology with ill-defined borders (figure 5C). Some cases showed scarce podoplanin-immunoreactive lymphatic endothelial cells (figure 2B), while others showed an abundance (figure 5A). One case was positive for both lymphatic mRNAs via FISH (figure 6A–C) as well as a lymphatic protein marker (podoplanin) via IF (figure 2B). The lymphatic marker profile was seen as a vessel-like structure with a potential lumen on IF (figure 3B) and elongated profiles on FISH (figure 6A–C). The other case, although positive for lymphatic mRNAs (figure 6D–I), was negative for podoplanin on IF. Further, one case was double positive for both LYVE-1/podoplanin (figure 6F) and LYVE-1/VEGFR-3 (figure 6I), while the other case was double positive for LYVE-1/podoplanin (figure 6C) but did not show any VEGFR-3 positivity. The case which was negative for podoplanin IF but positive for lymphatic mRNA was double positive for both LYVE-1/VEGFR-3 and for LYVE-1/podoplanin.
Supplemental material
Lymphatics of varying morphologies and sizes from 4 separate cases. (A) Case ID #3: a 68-year-old male patient with a failed graft. (B) Case ID #2: an 87-year-old female patient with a failed graft. (C) Case ID #1: an 88-year-old male patient with a failed graft. (D) Case ID #4: an 81-year-old male patient with a failed graft. Distinct podoplanin-positive lymphatics with different sizes, morphologies and clear lumen from four separate cases are shown in immunofluorescence images (A–D) at 63× magnification. The largest measured diameters of the lymphatics shown were as follows: (A) 10 and 20 µm, (B) 37 µm, (C) 54 µm, (D) 44 and 44 µm. All scale bars for this figure represent 10 µm.
Lymphatics of varying morphologies and sizes within a single case. Case ID # 1: the above immunofluorescence images were obtained from an 88-year-old male patient with a failed graft. Distinct podoplanin-positive lymphatics with different sizes, morphologies and clear lumen from one single case are shown (A–D) at 63× magnification. The largest measured diameters of the lymphatics shown were as follows: (A) 9 µm, (B) 33 µm, (C) 50 µm, (D) 84 µm. All scale bars for this figure represent 10 µm.
Additional unique lymphatic morphologies. (A) Case ID #2: an 87-year-old female patient with failed graft. (B and C) Case ID #1: an 88-year-old male patient with a failed graft. Immunofluorescence images are shown in A–C. Abundant podoplanin-positive lymphatics are seen at 20× magnification (A). Distinct podoplanin-positive lymphatics with different sizes and morphologies from a single case are shown (B and C) at 63× magnification. The largest measured diameters of the lymphatics shown at high-power were as follows: (B) 35 µm and (C) 39 µm. Scale bars for this figure represent (A) 50 µm and (B and C) 10 µm.
Fluorescence in situ hybridisation detection of lymphatic marker mRNAs. (A–C) Case ID #2: an 87-year-old female patient with a failed graft. (D–I) Case ID #5: a 30-year-old male patient with a failed graft. Probes recognising (A and D) podoplanin mRNA, (B, E, and G) LYVE-1 or (H) VEGFR-3 were used. (C, F and I) 4′,6-diamidino-2-phenylindole is also shown (blue). A merged image (C, F) illustrates podoplanin and LYVE-1 double positive regions (yellow). Another merged image (I) illustrates a LYVE-1 and VEGFR-3 double positive region (orange). Scale bars represent 30 µm.
Discussion
This is the first study to systematically investigate failed corneal grafts with neovascularisation to identify lymphatics using multiple techniques and to prove that haemangiogenesis and lymphangiogenesis are distinct entities. Furthermore, compared with previous work, we examined the morphology of podoplanin positive regions extensively ,to be confident of the presence of lymphatics, which we demonstrate to be separate from blood vessels. Our study provides for the first time multiple examples of lymphatics from the corneas of various patients , revealing a spectrum of findings including characteristic ‘luminal’ morphology. We have identified the presence of lymphangiogenesis in failed grafts with high-resolution images showing clear lumina on IF in addition to FISH images demonstrating lymphatic mRNAs in failed grafts.
Seo et al observed the presence of podoplanin positivity along with other lymphatic markers in acutely failed grafts, however, did not identify a clear lumen.15 Their criteria included graft failure with corneal oedema, which was often acute.15 Our study illustrated numerous podoplanin-positive profiles containing lumina that were morphologically consistent with lymphatic channels. The presence of chronic inflammation and neovascularisation may have contributed to the finding of a more mature morphology among our cases. Evidence of lymphatics in corneas with multiple pathologies, including graft failure has been reported by IHC.9 However, neither IF nor FISH findings were reported specifically in failed graft cases.9
Previous studies conducted by Cursiefen et al , Regina et al , Tshionyi et al and Seo et al established a role of lymphangiogenesis in pathological corneal conditions.7–9 15 Our study is the first study to use both lymphatic–endothelial and blood vessel specific markers to document the presence of lymphatic vessels and to prove systematically that lymphangiogenesis and haemangiogenesis occur separately in cases of graft failure with neovascularisation. Furthermore, ours is the first detailed report of a wide spectrum of morphologies of lymphatic vessels in graft failure, clearly distinct from blood vessels.
Cursiefen et al demonstrated the importance of lymphangiogenesis in pathological corneal conditions including graft failure.9 However, they did not seek to distinguish lymphangiogenesis from haemangiogenesis as in our study.9 Regina et al were crucial in establishing that lymphangiogenesis appears to only occur in the presence of haemangiogenesis.8 Unlike our study, simultaneous exploration of lymphatic and blood vessel markers was not performed.8 Additionally, while we systematically investigated corneal graft failure, Regina et al focused on a variety of corneal pathologies including several cases of graft failure.8 Similarly, while Tshionyi et al provided another significant article in investigating the importance of lymphangiogenesis in multiple corneal pathologies, few graft failure cases were included—none found to be positive for lymphatic markers making it difficult to infer any conclusions regarding the role of lymphatics in graft failure in their study.7 Finally, although Seo et al provided much useful quantitative data regarding the presence of various lymphatic biomarkers, they did not focus on morphology of the lymphatics or their separation from blood vessels. Finally, the inclusion criteria used by Seo et al ensured that only cases of acute rejection were used and not cases with ‘natural graft loss’.15 Thus, while all the aforementioned studies were crucial in establishing the importance of lymphangiogenesis in corneal pathology and corneal graft failure, our study is the first to systematically investigate lymphangiogenesis in neovascularised corneal graft failure cases while qualitatively demonstrating that lymphangiogenesis and haemangiogenesis are occurring as separate processes spatially.
It is widely accepted that human corneas do not contain lymphatic channels, except under pathological conditions. This was confirmed in our study by the IF analysis of healthy corneas, which were not found to contain lymphatics. Any lymphatic-specific protein or mRNA expressed in the corneal stroma would be considered abnormal. Markers including podoplanin, LYVE-1 and VEGFR-3 are commonly used to assess for lymphatic channels. CD31, a common and traditional marker of blood vessels, is weakly expressed on lymphatics.18 Podoplanin can be selectively targeted by the antibody D2-40, which we used in our study.17 Podoplanin is specific to lymphatic endothelium and is absent from blood vessel endothelium.17 Conversely, LYVE-1 has also been found to be expressed in some macrophages and blood vessels, such as liver sinusoids.18 Similarly, VEGFR-3 can be found in some blood vessels, including inflammation-induced angiogenesis.18 In our study, we sought to observe both distinct blood and lymphatic vessels simultaneously using IF. Since LYVE-1 and VEGFR-3 can be found in blood vessels, while podoplanin is only found in lymphatics, we chose to use only podoplanin in our study. This allowed us to be sure that observed lymphatics were not actually blood vessels. The fact that the podoplanin protein antibody coincided with structures consistent with lymphatics further supports the presence of lymphatics in observed cases. The combined use of IF detection of lymphatic protein and FISH detection of lymphatic mRNAs in two of our cases adds an extra layer of specificity to our results. Our IF experiments may be seen to be limited by utilisation of only a single lymphatic marker, podoplanin. However, a recent ‘Consensus Statement on the Immunohistochemical Detection of Ocular Lymphatic Vessels’ suggested that the use of a single lymphatic marker is sufficient for areas in which the presence of lymphatics has been well established, including the inflamed cornea.19 Schroedl’s consensus paper mentions podoplanin, LYVE-1 and VEGFR-3 as markers used in identifying lymphatics in the eye in general. While they suggested using a panel of at least two markers to identify lymphatics in the eye, they state that ‘this is not relevant for sites where the existence of lymphatic vessels is already well established as is the case for pathologically vascularized corneas’.19 Thus, since we investigate pathologically vascularised corneas, a single marker was selected. Unlike LYVE-1 and VEGFR-3 which are found in blood vessels, podoplanin is not found in blood vessels and was therefore the preferred marker in order to clearly distinguish lymphatics from blood vessels.17 18
There is little in the literature comparing the sensitivity and specificity of immunoperoxidase (used in immunohistochemical methods and commonly in diagnostic pathology) versus IF (used mainly in research). However, IF allowed the use of confocal microscopy with better resolution and labelling with multiple lymphatic and blood vessel endothelial cell markers. IHC allowed us to screen for the presence of suspected lymphatics. IF confirmed the presence of lymphatics with better resolution, demonstrating that these lymphatics are distinct from any blood vessels present in the cornea. Stacks of confocal microscopy images provided clear three-dimensional images of lymphatic vessels that were spatially distinct from blood vessels, providing evidence that these were not disguised blood vessels. In this study, we observed distinct podoplanin-antibody immunoreactive lymphatics with many different morphologies. Perhaps, the observed morphologies represent lymphatics at different stages of lymphangiogenesis within the adult cornea. Elongated structures may represent collapsed lymphatic vessels since lymphatic vessels have a relatively thin endothelial layer. Vessels may have collapsed in the process of dehydration during formalin fixation. Conversely, these elongated profiles could represent relatively immature lymphatics, with a less developed, more collapsible endothelium. Lumen-like profiles observed may represent histological sections that were oblique and not cut precisely perpendicular to the lymphatic lumen and therefore did not include the entire circumference surrounding the lumen. Another explanation may be that transection of a vessel branchpoint may not show a single, complete lumen. Similar CD31 immunoreactive vessel morphologies were seen and support the above reasons regarding lymphatics. The fact that VEGFR-3 positivity was seen on FISH in one case and not the other may be due to heterogeneity of the lymphatics observed. Indeed, lymphatic endothelium markers can be expressed differentially in varying functional states.18 A better understanding of the individual roles and interplay between different lymphangiogenic markers may help clarify these variable staining patterns. Another possibility is that elongated profiles represent lymphatics that are not fully developed. In support of this idea, Ohtani et al observed that lymphatic endothelial cells may form lines of single cells before arranging into channels.20 These elongated profiles may therefore represent linear arrangements of lymphatic endothelial cells. Although one of the cases was negative for podoplanin protein, it was positive for podoplanin, LYVE-1 and VEGFR-3 mRNAs in FISH. The fact that two areas of double-positive lymphatic mRNA were seen on FISH increases the likelihood that this sample contained lymphatics. If lymphatics were present in this case, perhaps they were missed due to a limitation of the sensitivity of the D2-40 podoplanin antibody as a lymphatic marker. If the positive profile seen in FISH did, as suggested, represent an immature lymphatic channel, perhaps it was difficult to visualise on IF analysis.
Some insight into lymphangiogenesis may be gleaned from our study in which we observed varying distributions and morphologies of lymphatic vessels. Some of our cases included scattered, single examples of lymphatic vessels. Other cases showed multiple examples of lymphatics or structures that may represent a network of interconnected lymphatic channels. While it is commonly thought that lymphatics are embryologically derived from venous endothelium, lymphangiogenesis in the adult cornea may occur in a different fashion, especially given that lymphangiogenesis may not follow the same mechanism in all organs.21 Additionally, recent studies in mouse models have suggested that corneal lymphangiogenesis may be transient.22 23It has been suggested that corneal lymphangiogenesis may develop in a spatially contiguous manner from the limbal lymphatics.24 Our finding of cases that demonstrated abundance of potential lymphatic networks may support this model. Our observation of other cases with single lymphatic vessels scattered sparsely throughout the cornea with no progression from the limbus may not support this model. However, assuming that lymphangiogenesis is both a transient and chronic process in these cases that are likely undergoing constant allorejection, it is possible that early lymphatic networks that developed from the limbus have since regressed, leaving only some remaining lymphatic channels scattered throughout the cornea. Therefore, lymphangiogenesis may be a very dynamic process in corneal graft failure. A study by Maruyama et al found that CD11b+ macrophages may be able to form lymphatic channels de novo within the corneal stroma, with no connexion to limbal lymphatics.25 Given our data, as well as the presence of inflammation within all our cases, this model for lymphangiogenesis seems plausible. Consistent with this, Seo et al demonstrated a significant increase of CD11b+ macrophages in graft failure with herpetic keratitis.15 However, the pathophysiology may vary between acute and chronic conditions. While we attempted to elucidate the original pathologies underlying corneal graft failure in our samples, this information was mostly unavailable. Our observation that lymphatics may be present as sparse channels or more extensive networks in graft failure suggests that there may be multiple mechanisms responsible for lymphangiogenesis, depending on the underlying circumstances. Additional studies are needed to further understand mechanisms of lymphangiogenesis in corneal graft failure and other pathologies. This will lead to a further clarification of the mechanism of lymphangiogenesis under pathological conditions.
Our study is limited by the fact that while we did search for more extensive clinical information, much of this information was not available to us. Future studies may investigate more in-depth clinical histories in cases of graft failure with distinct lymphangiogenesis and haemangiogenesis and possible clinical correlations. Lymphatics represent the afferent pathway of the immune system, bringing antigen-presenting cells to draining lymph nodes.3 This immune response/rejection is then carried out by inflammatory cells carried back to the diseased tissue through the efferent pathway of blood vessels.3 Inflammatory infiltrates were seen in every case we examined, suggesting that both the afferent and efferent pathways must have been intact in order to induce allorejection. Our findings support a role for lymphatics in graft rejection and failure, emphasising the promising future of therapies that target lymphatics.
First-line management of corneal graft rejection currently centres around the use of topical corticosteroids.3 Alternative treatment includes immunosuppressive agents such as ciclosporin and tacrolimus,3 which are generally associated with more serious adverse events due to systemic immunosuppression. Corticosteroids have been found to reduce lymphangiogenesis in mouse models.26 27 However, in high-risk patients with pre-existing stromal neovascularisation or history of graft rejection, the efficacy of first-line management is nearly halved.3 This demonstrates the need to establish more effective treatments in these high-risk patients.
Novel treatment options are currently being investigated to target lymphatic vessels in failing grafts. Such antilymphatic based therapies include the use of podoplanin-neutralising antibodies,28 inhibition of ITGA-9 induced lymphatic valve formation,29 photodynamic therapy with verteporfin,30 sVEGFR-2, a VEGF-C lymphangiogenesis factor antagonist31 and an antibody directed to VEGFR-3.32 However, these studies were all performed in mice. No clinical studies, as far as we are aware, have investigated treatment that specifically targets lymphatics and not blood vessels in graft failure. Selective suppression of lymphangiogenesis is important because suppression of angiogenesis has been shown to impair wound healing and can induce graft failure due to wound rupture after removal of sutures.33 Selective lymphangiogenesis inhibition can block the afferent pathway of immune rejection and has been found to strongly improve graft survival, regardless of angiogenesis suppression.31 33
Our finding of lymphatics in failed corneal transplants highlights the importance of developing therapies that target lymphatic vessel growth. Perhaps the presence of lymphatic vessels can also be used as a prognostic marker, allowing certain patients to receive more personalised therapy. For instance, one study found that in vivo confocal microscopy can be used to detect corneal lymphatics in mice.34 The authors suggested that this modality may be used in the near future to non-invasively assess the risk of graft failure.34 Likewise, microscopic optical coherence tomography was also found to be capable of detecting lymphatics in murine corneas.35
Our findings of lymphangiogenesis within failed corneal grafts with neovascularisation stresses the importance of developing new tools including therapies and imaging modalities directed towards lymphatics in order to combat graft failure. It is our hope that as our understanding of the role of lymphatics progresses, we will see much needed improvements in graft survival.
Acknowledgments
We acknowledge the Human Eye Biobank for Research, St. Michael's Hospital for providing invaluable tissue for our studies www.humaneyebank.com
References
Footnotes
Contributors MAD, XZ, YG and EG, completed all experimental components and contributed to interpretation of results. MAD, SWSC, YG, YY and NG contributed to experimental design and interpretation of results. MAD, SWSC, YY and NG wrote the paper.
Funding We are grateful to the Harcharan & Prem Singh Foundation and Canadian Institutes of Health Research (MOP119432; YHY, NG).
Competing interests None declared.
Patient consent Not required.
Ethics approval St. Michael's Hospital Research Ethics Board.
Provenance and peer review Not commissioned; externally peer reviewed.
Linked Articles
- At a glance