Article Text
Abstract
Purpose To explore the risk factors for ophthalmic artery (OA) stenosis and occlusion after intra-arterial chemotherapy (IAC) with selective ophthalmic artery catheterisation (OAC) in the treatment of retinoblastoma.
Design Retrospective, single centre case-control study.
Methods The study was conducted including consecutive patients with unilateral or bilateral intraocular retinoblastoma undergoing IAC between June 2016 and June 2019 with a follow-up time of 4 years. Main outcomes are rate of IAC-induced OA occlusion and OA diameter.
Results 346 attempted OAC infusions were successful. The total incidence of OA occlusion was 15.89%. The occlusion and control groups were similar in patients’ age, sex and disease stage. Median OA diameter was 0.49 mm in those with OA occlusion, and 0.66 mm in those without occlusion. In the occlusion group, the OA diameter difference was significantly larger between the first IAC and the final IAC (0.22mm vs 0.12mm, p=0.001). In both groups, the median number of IAC treatments was 3. Multivariate Cox regression models included initial OA diameter (OR: 0.005, p=0.001), ratio of OA orifice diameter differences between first and last IAC to the initial OA orifice diameter (OR: 4.661, p=0.003), and number of IAC (OR: 1.538, p=0.042) as clinical features significantly associated with OA occlusion.
Conclusions The OA diameter at first IAC treatment, the ratio of OA orifice diameter differences between first and last IAC to the initial OA orifice diameter and total number of IAC treatments may be three main clinical predictors for OA occlusion after IAC for retinoblastoma.
- retina
- Anatomy
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. All free text entered below will be published.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
Retinoblastoma accounts for only 3% of childhood cancer (which itself is rare) but is the most common childhood intraocular retinal malignancy.1 China has the highest number of cases in the world, with more than 70% of diagnosed eyes in advanced stages.2 Beginning as a tiny intraretinal tumour, it may grow into the eye, spread to the brain and cause remote metastasis in 1–2 years.3 If left untreated, retinoblastoma and metastases are potentially lethal. Therefore, the primary goal of treatment is to maintain life, with ocular survival, visual preservation and quality of life as secondary goals.1 In the past decade, intra-arterial chemotherapy (IAC) has been used increasingly in the clinical management of retinoblastoma given its success rate for ocular survival in advanced or refractory tumors.4–8 Many studies have demonstrated the effectiveness of this approach, even in advanced-stage cancers.9–11 In general, most patients receive treatment at 3-monthly sessions. So far, no fatal or life-threatening complications have been reported.12 Although IAC is considered to be relatively safe, this surgery could be followed by retinal vein occlusion, artery occlusion or intraocular haemorrhage.13 The purpose of this case-control study was to explore the risk factors for ophthalmic artery (OA) stenosis and occlusion after IAC with selective ophthalmic artery catheterisation (OAC) in the treatment of retinoblastoma. The fluorescein angiogram showed a marked delay in the filling of the choroidal circulation when OA occlusion occured.14
Methods
Group creation and oversight
A retrospective single centre case-control study was conducted, including the records of consecutive patients with unilateral or bilateral intraocular retinoblastoma undergoing IAC between June 2016 and June 2019 with a follow-up time of 4 years. The clinical stages of patients were classified by International Intraocular Retinoblastoma Classification (IIRC). Exclusion criteria were diffuse infiltrating retinoblastoma, anterior chamber invasion, secondary glaucoma, vitreous haemorrhage, optic nerve infiltration, diffuse choroidal infiltration, scleral infiltration, extraocular disease and intracranial metastatic disease apparent on gadolinium-enhanced MRI of the head and orbits. The advanced stages referred to groups D and E. Patients who had undergone procedures performed with alternative routes for the delivery of OAC including use of a balloon-assisted drug infusion were also excluded. Comparative analysis was performed with a control group who had undergone OAC but did not develop OA stenosis and/or occlusion. Group categorisation was based on selective internal carotid artery (ICA) angiography, with opacification of the OA indicating no occlusion (control) and a lack of opacification indicating occlusion (figure 1). An alternative approach was used to complete the procedure, including external carotid artery (ECA) routes and ICA balloon application.
Super-selective angiographic microcatheter series of the ophthalmic artery (OA). (A) Regular appearance of the OA. (B) Meningeal collaterals that may impede intra-arterial chemotherapy. Lateral digital subtraction angiogram showing super-selective catheterisation of the OA and contrast injection without the presence of contrast medium reflux into the internal carotid artery.
Informed consent was not required in view of the retrospective study design.
Surgical procedure
IAC was performed by oncologists using sterile techniques under general anaesthesia. A 4F paediatric guide microcatheter (diameter 0.97 mm) was used to enter the common femoral artery, then the sheath was put in place and the ipsilateral ICA was inserted. The anatomic structure of the OA was visualised by serial angiography and superselective Prowler 10 microcatheter (diameter 0.57 mm) inserted into the OA orifice. Superselective injection via microcatheter was performed to check correct placement and to assess the amount of reflux (if any) into the ICA before chemotherapy was administered. Chemotherapy drugs were administered at dosage increasing with age and tumour size (melphalan at 3.5 to 7.5 mg; topotecan at 1 mg; carboplatin at 20 mg). Repeat final lateral arteriography of the ICA or the common carotid artery (depending on the route of administration) was conducted immediately after surgery to exclude complications such as vasospasm, embolism or dissection. If an adequate choroidal blush was unattainable via the direct catheterisation of the OA, alternative routes of IAC delivery were sought via anastomoses with the ECA.8 15 16
Statistical analysis
Categorical variables were divided into absolute and relative frequencies and compared using the χ2 test or Fisher’s exact test (if the χ2 test was not applicable), while quantitative variables were presented as means and medians with SD using an unpaired t-test or Wilcoxon rank sum test (if t-test was not applicable). In the univariate analysis, crude analysis was performed to identify potential risk factors. After potential risk factors of OA stenosis and occlusion were selected, multivariate logistic regression and Cox regression analyses with three selection procedures (forward, backward and stepwise) were developed to further select the best-fit model. Receiver operating characteristic (ROC) curves were generated to determine the threshold of predictors for further survival analysis. All statistical tests were two-sided unless otherwise specified and a p value less than 0.05 indicated statistical significance. All analyses were performed using SPSS software (V.22.0, IBM, Armonk, New York, USA) and RMS packages in R V.3.4.1 (Vienna, Austria; http://www.R-project.org/).
Outcome variables
The primary outcome analysed was OA diameter. Other variables such as the incidence of OA stenosis, OA diameter at ostial position, angles between OA and ICA, number of IAC treatments and incidence of enucleation were also evaluated.
Results
Patients’ demographics
The baseline demographic characteristics are presented in table 1. There were 352 attempted OAC infusions in 107 retinoblastoma tumours of 105 consecutive patients between June 2016 and June 2019, 346 of which were successful. In two of those patients, both eyes were successfully treated. The total incidence of OA stenosis and occlusion was 15.89% (17/107). Median diagnosis age of the 105 patients was 24 months (range 2.1 months to 83.9 months). Comparative analysis between the occlusion group and the control group showed no significant difference in diagnosis age between the two cohorts (mean 22.29 months vs 24.40 months, p=0.55), sex (10 (18.5%) female patients vs 44 (81.5%) female patients, p=0.99), IIRC stage (group C: 0.0% vs 8.9%, p=0.61; group D: 11.8% vs 30.0%, p=0.141; group E: 88.2% vs 61.1%, p=0.165) or number of IAC treatments (mean 3.71±1.26 vs 3.10±1.32, p=0.084).
Baseline demographic and clinical characteristics of the patients.
OA diameter and ocular survival rate
The OA diameter at first IAC was 0.66 mm (range 0.22–1.03 mm) in all patients, 0.49 mm (range 0.22–0.67 mm) in the occlusion group and 0.66 mm (range 0.37–1.03 mm) in the control group (table 1). Using univariate Cox regression analysis, we confirmed a significant difference between the two groups in terms of OA diameter at the first treatment (p=0.02) (table 2). A significant difference between the two groups was also found in the ratio of diameter before final IAC to the initial diameter of OA at ostial position (p=0.001). The OA diameter was reduced between the first and final IAC treatments, and this difference was larger in the occlusion group than in controls (0.22 vs 0.12, p=0.001). The OA diameter at the ostial position also decreased from first to final IAC, and this difference was again larger in the occlusion group than in controls (0.14 vs 0.099, p=0.001). The OA diameter also differed between the two groups at the last treatment as the control group had larger OA diameter (p<0.05). The ratio of diameter differences between first and last IAC to the initial OA diameter was 0.37 (±0.21) in the occlusion group and 0.17 (±0.15) in the control group. The corresponding ratio at ostial position was 0.35 (±0.17) in the occlusion group and 0.19 (±0.15) in the control group. Four-year ocular survival rate was 80.0% (±9.1%) in group E patients with an OA diameter larger than 0.65 mm. Four-year ocular survival rate was 36.3% (±14.2%) in group E patients with an OA diameter less than 0.65 mm (online supplemental table 1).
Supplemental material
Univariate predictors of ophthalmic artery stenosis and occlusion: Cox regression analysis
IIRC stages and number of IAC
IAC was performed in 90 (85.7%) eyes and others were interrupted because of transient OA spasm ECA (seven cases; 6.5%), OA occlusion (seven cases; 6.5%) or congenital stenosis of the OA or other retinal blood supply (three cases; 2.8%). In the occlusion group, no patient was diagnosed with group C disease, 2 (11.8%) patients were diagnosed with group D and 15 (88.2%) patients were diagnosed with group E according to the IIRC diagnostic criteria. In the control group, 8 (8.9%) patients were diagnosed with group C, 27 (30.0%) patients were diagnosed with group D and 55 (61.1%) patients were diagnosed with group E (table 1). In the occlusion group, 6 (40.0%) patients with group E underwent enucleation during the study period. In the control group, these numbers were1 (3.7%) with group D and 30 (54.5%) with group E (table 1). In both groups, the median number of IAC treatments was 3 (mean, 3.71 vs 3.10, occlusion group vs control group). In the control group, 4 patients (4.4%) received a total number of 6 IAC, 3 patients (3.3%) received 5 IAC sessions, 36 patients (40.0%) received 4 IAC sessions, 19 patients (21.1%) received 3 IAC sessions, 14 patients (15.6%) received 2 IAC sessions and 15 patients (16.7%) received 1 IAC session. While in the occlusion group, 1 patient (5.9%) received a total number of 7 IAC, 1 patient (5.9%) received 6 IAC sessions, 7 patients (41.2%) received 4 IAC sessions, 6 patients (35.3%) received 3 IAC sessions and 2 patients (11.8%) received 2 IAC sessions (table 1).
Predictors of OA occlusion and ocular survival
ROC analysis indicated two categories of OA diameter, at 0.65 mm or less (group SN, ‘SN’ refers to smaller diameter in online supplemental figure 1) or over 0.65 mm (group LN, ‘LN’ refers to larger diameter in online supplemental figure 1). In group LN, OA occlusion and ocular survival were similar (online supplemental table 1) in both groups D and E, with zero OA occlusion at first year .
Supplemental material
Within patients in group LN with group E disease, a significant difference in OA diameter was found between the occlusion and control groups (table 2 and online supplemental table 2). In patients with group D disease, the rate of OA occlusion-free survival and ocular survival was found to be 90.0% (SD, 8.7%) at 1 year and 75.8% (SD,15.6%) at each of the years 2–4. In SN patients with group E disease, the rate was 85.6% (SD, 6.7%) at 1 year, 71.6%(SD, 9.3%) at 2 years, and 36.3% (SD, 14.2%) at 3 and 4 years. The ocular survival rate within group SN at 2 years was 80.0% (SD, 17.9%) in patients with group D disease and 64.5% (SD, 10.8%) in those with group E disease (online supplemental table 2).
No significant difference was found between the occlusion and control groups in either the time to OA stenosis or time to occlusion in patients with group D disease (p=0.063) but these time periods did differ significantly in those with group E disease (p=0.0008) (online supplemental table 2). Kaplan-Meier survival curves demonstrate the primary outcome of OA stenosis and occlusion on the basis of initial OA diameter among first to last IAC treatments (p=0.0018) (figure 2).
Cumulative Incidence of ophthalmic artery (OA) stenosis and occlusion after each intra-arterial chemotherapy (IAC) treatment, stratified by initial OA diameter Kaplan-Meier survival curves demonstrate the primary outcome of OA stenosis and occlusion on the basis of initial OA diameter among first to last IAC treatments (p=0.0018). LN, large diameter; SN, smaller diameter.
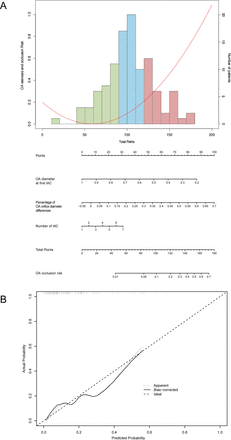
The Cox proportional-hazards model indicated that OA diameter is a univariate predictor of OA stenosis and occlusion (p=0.020, 95% CI 0.000 to 0.418) (table 2). No significant difference was found between the two groups in terms of sex, age at first IAC, diameter of OA at ostial position, angles between OA and ICA or number of IAC treatments. Multivariate Cox regression models analysed with three selection procedures (forward, backward and stepwise) were developed to further select the best-fit model (table 2). A statistical significance level of 0.20 was used to select variables into the model. After comparing the models from each procedure, the final model was from the forward selection process with p<0.2,included OA diameter at first IAC (OR, 0.005, 95% CI 0.001 to 0.172, p=0.001), ratio of OA orifice diameter differences between first and last IAC to the initial OA diameter at ostial position (OR, 4.661, 95% CI 3.600 to 31.741, p=0.003), and number of IAC (OR, 1.538, 95% CI 1.0.15 to 2.232, p=0.042) as the three clinical features significantly associated with OA occlusion (table 2). The nomogram developed from the multivariable model for predicting OA occlusion is shown in figure 3A. This three-factor OA-occlusion risk score assigned each factor a corresponding point value based on the correlation of each factors with OA occlusion. The nomogram demonstrated relatively good performance in estimating the OA-occlusion risk, with a C index of 0.772 (95% CI 0.65 to 0.89). Besides, the calibration plot graphically showed the consistency of OA occlusion between the risk predicted by the nomogram and actual risk confirmed by histopathological examination (figure 3B).
Nomogram to predict the risk of ophthalmic artery (OA) occlusion in patients with retinoblastoma with intra-arterial chemotherapy (IAC) treatments. (A) Risk curve refers to the OA occlusion possibility based on the risk scores of different risk factors. Histogram shows the distribution of risk factors scores in this cohort. The green bar refers to low risk, the blue bar refers to medium risk and the red bars refers to high risk. (B) The calibration plot showed the actual probability of OA occlusion over the predicted risk probability.
Discussion
The treatment of retinoblastoma requires a delicate balance between the effectiveness and toxicity combined with globe and vision survival.10 Successful use of IAC requires skill and experience. In previous clinical experience, the use of IAC may be impeded by three conditions: (1) significant collaterals to meningeal arteries, (2) technical failure of OA catheterisation or (3) retinal blood supply from collaterals other than the OA.17 Stathopoulos et al 18 had suggested that the catheterisation of OA should be performed through an ostial position or an ECA route to avoid the risk of choroidal complications.
The eye receives a dual blood supply from both internal and external circulation, usually involving passage through the OA, in which circumstance the OA is not visible on an angiogram of the ICA.14 An overall assessment of complications with 5 years of patient follow-up indicated 2% incidence of OA occlusion.4 In the present study, the success rate of IAC surgery was 98.3% during this period and the total incidence of OA stenosis and occlusion was 15.89% (17/107). Three conditions (transient stenosis spasm, congenital stenosis and OA thrombosis with subsequent occlusion, if alternative routes were not possible) carried a risk of IAC termination. Previous research has shown interaction between IAC drugs and the vascular endothelium and monocytes, which would lead to vessel wall endothelial cell changes, leukostasis and vessel occlusion.19 Sweid et al 14 found that earlier this year, local associated factors of chemotherapy and selective microcatheterisation of OA are essential factors in the development of OA thrombosis. Among these, OA occlusion had a major role in the potential damage of vision-threatening adverse effects.
In this retrospective case-control study, baseline characteristics such as age at first IAC and gender were found to be unrelated to OA occlusion. Anatomical factors such as the OA diameter and the calibre of OA at its orifice, angles between the OA and ICA, and the difference between OA diameter at the first and final IAC were also evaluated. Patients with initial OA diameter less than 0.65 mm had a relatively high likelihood of OA stenosis and occlusion after IAC treatment, particularly those with disease group E. Ravindran et al 20 had reported the lacking of high-level evidence with adequate follow-up regarding the long term of IAC and its impact on retinoblastoma. After 4 years of follow-up in this study, only 36.3% (±14.2%) patients were free of OA occlusion and ocular enucleation in group E with an OA diameter less than 0.65 mm. These findings suggested that global salvage rate in group E patients might become worse when initial OA diameter of patient is less than 0. 65 mm. The Cox regression analysis results also indicated that OA diameter prior to IAC, the ratio of OA orifice diameter differences between first and last IAC to the initial OA diameter at ostial position and total number of IAC treatments may be three predictors of OA occlusion after this procedure for retinoblastoma. As shown in the nomogram, patients with fewer than 90 points were considered to have low risk of OA occlusion. Patients with points between 90 and 120 points were medium-risk patients and those with points larger than 120 points were considered to have high risk of OA occlusion. When the OA diameter decreased to 65% of its initial value, IAC surgery became complicated, and options such as ECA pathway or balloon-assisted drug delivery were considered.
Limitations
In this retrospective single centre case-control study, there were three sources of bias here: a retrospective study (in which methodology could not be controlled and masking was not possible), a single centre (reflecting only results from that clinical system) and Han People (representing results only from one part of the wider population), which may lead to existence of statistical bias. The study also only included patients with retinoblastoma in groups C, D and E, thus lacking data representative of patients with retinoblastoma in groups A and B.
Conclusion
OA diameter prior to IAC, the ratio of OA orifice diameter differences between first and last IAC to the initial OA orifice diameter and total number of IAC treatments may be three predictors of OA stenosis and occlusion after this procedure. For patients with OA diameters less than 0.65 mm, attention should be paid to the possibility of OA stenosis and occlusion as treatment progresses. The nomogram model for OA occlusion may act as a supportive tool for optimised clinical evaluation and treatment decisions.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. All free text entered below will be published.
Ethics statements
Patient consent for publication
Ethics approval
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Ninth People’s Hospital affiliated with Shanghai Jiao Tong University School of Medicine. And the number of the approval is SH9H-2020-T331-1.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
MZ, XW and SJ contributed equally.
Contributors Conception and design: XF, RJ, JF. Collection and assembly of data: MZ, XW, SJ. Data analysis and interpretation: All authors. Manuscript writing: All authors. Final approval of manuscript: All authors. Accountable for all aspects of the work: All authors.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Linked Articles
- Highlights from this issue