Abstract
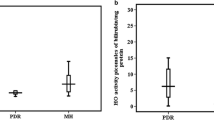
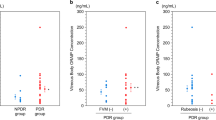
Lipid peroxide (LPO) levels, as determined by high-performance liquid chromatography (HPLC) and by the thiobarbituric acid (TBA) method, and myeloperoxidase (MPO) activity in vitreous of patients vitrectomized because of proliferative diabetic retinopathy were compared with LPO levels and MPO activity in vitreous of patients with no vitreoretinal proliferation. Both LPO levels and MPO activity were significantly elevated in the vitreous of patients with fibrovascular vitreoretinal proliferations secondary to diabetes. The TBA method produced higher values for LPO levels than did the HPLC method. The correlation between the two methods was 0.94. Our results suggest that both oxygen-free radicals and inflammation-related reactions can participate in the pathogenesis of diabetic retinopathy.
Similar content being viewed by others
References
Augustin AJ, Lutz J (1991) Intestinal, hepatic and renal production of thiobarbituric acid reactive substances and myeloperoxidase activity after temporary aortic occlusion and reperfusion. Life Sci 49:961–968
Augustin AJ, Breipohl W, Böker T, Wegener A (1992) Evidence for the prevention of oxidative tissue damag in the inner eye by vitamin E and vitamin C. Ger J Ophthalmol 1:394–398
Borgeat P, Samuelsson B (1979) Transformation of arachidonic acid by rabbit polymorphonuclear leukocytes. J Biol Chem 254:2643–2646
Bradley PP, Priebat DA, Christensen RD, Rothstein G (1982) Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol 78:206–209
Collier A, Jackson M, Dawkes RM, Bell D, Clarke BE (1988) Reduced free radical activity detected by decreased diene conjugates in insulin-dependent diabetic patients. Diabetic Med 5:747–749
Doly M, Droy-Lefaix MT, Bonhomme B, Braquet P (1986) Effect of Ginkgo biloba extract on the electrophysiology of the isolated retina from diabetic rat. Presse Med 15:1480–1483
Esterbauer H, Lang J, Zadravec S, Slater TF (1984) Detection of malonaldehyde by high-performance liquid chromatography. Meth Enzymol 105:319–328
Gimbrone MA, Brock AF, Schaffer AI (1984) Leukotrien B4 stimulates polymorphonuclear leukocyte adhesion to cultured vascular endothelial cells. J Clin Invest 74:1552–1555
Goldstein IM, Roos D, Kaplan HB, Weissmann G (1975) Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest 56:1155–1163
Granger DN, Rutili G, McCord JM (1981) Superoxide radicals in feline intestinal ischemia. Gastroenterology 81:22–29
Gross JI, Moscatelli D, Rifkin DB (1983) Increased capillary endothelial cell protease activity in response to angiogenic stimuli in vitro. Proc Natl Acad Sci USA 80:2623–2627
Hartmann JR, Robinson JA, Gunnar RM (1977) Chemotactic activity in the coronary sinus after experimental myocardial infarction: effects of pharmacologic intervention on ischemic injury. Am J Cardiol 40:550–555
Iyer GYN, Islam DMF, Quastel JH (1961) Biochemical aspects of phagocytosis. Nature 192:535–541
Jennings PE, Scott NA, Saniabadi AR, Belch JJ (1992) Effects of gliclazide on platelet reactivity and free radicals in type II diabetic patients: clinical assessment. Metabolism 41 [Suppl 1]:36–39
Jos J, Rybak M, Patin PH, Robert JJ, Boitard C, Thévenin R (1990) Antioxidant enzymes in insulin-dependent diabetes in the child and adolescent. Diabet Metab 16:498–503
Lucchesi BR, Mullane KM (1986) Leukocytes and ischemia-induced myocardial injury. Annu Rev Pharmacol Toxicol 26:201–204
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid. Anal Biochem 95:351–358
Reed PW (1969) Glutathione and the hexose monophosphate shunt in phagocytizing and hydrogen peroxide-treated rat leukocytes. J Biol Chem 2459–2464
Saugstad OD, Rognum TO (1988) High postmortem levels of hypoxanthine in the vitreous humor of premature babies with respiratory distress syndrome. Pedriatics 81:395–398
Sbarra JA, Karnowsky ML (1959) The biochemical basis of phagocytosis. J Biol Chem 234:1355–1362
Schacterle GR, Pollack RL (1973) A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal Biochem 51:654–655
Simonelli F, Pensa M, Teramo P, Amicone A, Russo P, Perillo F, Cotticelli L (1990) Hydrogen peroxide in the aqueous humor and cataract formation in human diabetes. Boll Soc Ital Biol Sper 66:879–885
Srivastava SK, Ansari NH, Liu S, Izban A, Das B, Szabo G, Bhatnager A (1989) The effect of oxidants on biomembranes and cellular metabolism. Mol Cell Biochem 91:149–157
Thérasse J, Lemonnier F (1987) Determination of plasma lipoperoxides by high-performance liquid chromatography. J Chromatogr 413:237–241
Weller M, Wiedemann P, Moteh H, et al. (1989) Transferrin and transferrin receptor expression in intraocular proliferative disease. APAAP-immunolabeling of retinal membranes and ELISA for vitreal transferrin. Graefe's Arch Clin Exp Ophthalmol 227:281–286
Weller M, Clausen R, Heimann K, Wiedermann P (1990) Iron-binding proteins in the human vitreous: Lactoferrin and Transferrin in Health and in Proliferative intraocular disorders. Ophthalmic Res 22:194–200
Weller M, Heimann K, Wiedemann P (1990) The pathogenesis of vitreoretinal proliferation and traction: a working hypothesis. Med Hypotheses 31:157–159
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Augustin, A.J., Breipohl, W., Böker, T. et al. Increased lipid peroxide levels and myeloperoxidase activity in the vitreous of patients suffering from proliferative diabetic retinopathy. Graefe's Arch Clin Exp Ophthalmol 231, 647–650 (1993). https://doi.org/10.1007/BF00921959
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00921959