Abstract
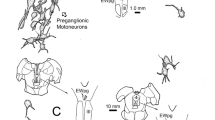
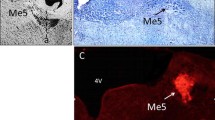
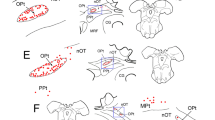
The peripheral nervous system is classically separated into a somatic division containing both afferent and efferent pathways and an autonomic division composed of efferents only. The somatic afferent division is divided in A- and B-neurons. The B-neurons are supposed to be autonomic afferents as part of a reflex system involved in homeostasis. Recent data obtained by neuronal tracing and immunohistochemical experiments concerning the eye related peripheral nervous system endorse the existence of these peripheral reflex systems. Somatic afferents of trigeminal origin synaptically innervate parasympathetic neurons in the pterygopalatine ganglion. This probably represents a pathway mediating autonomically regulated ocular activity in response to sensory stimulation. In addition, it has been hypothesized that trigeminal sensory nerve fibres have an efferent function in response to noxious stimuli e.g. the ocular injury response. Sympathetic nerve fibres originating in the superior cervical ganglion course through the trigeminal and pterygopalatine ganglion without forming direct synaptic contacts. These fibres, however, contain clusters of vesicles suggesting some kind of interneural communication. Parasympathetic nerve fibres of pterygopalatine origin course through the ciliary ganglion. These nerve fibre terminals also contain clusters of vesicles without direct synaptic contacts. Experimental data concerning the distribution of neuropeptides revealed a more detailed knowledge of the anterior eye segment innervation. These experimental data are subject to some debate. The pros and cons of different techniques are discussed. Neural circuits regulating IOP have long been postulated. The possible role of peripheral reflex systems in the regulation of IOP is discussed.
Similar content being viewed by others
References
Ruskell GL. An ocular parasympathetic nerve pathway of facial nerve origin and its influence on intraocular pressure. Exp Eye Res 1970; 10: 319–33.
Carpenter MB. Gross anatomy of the brain. Core text ofneuroanatomy. Baltimore: Williams & Wilkins, 1978: 15–44.
Brodal A. The cranial nerves. Neurological anatomy in relation to clinical medicine. New York/Oxford: Oxford University Press 1981: 448–578.
Windle WF. Non-bifurcating nerve fibers of the trigeminal nerve. J Comp Neurol 1926; 40: 229–40.
Olszewski J. On the anatomical and functional organization of the spinal trigeminal nucleus. J Comp Neurol 1950; 92: 401–13.
Arvidsson J, Grant G. Further observations on transganglionic degeneration in trigeminal primary sensory neurons. Brain Res 1979; 162: 1–22.
Pfaller K, Arvidsson J. Central distribution of trigeminal and upper cervical primary afferents in the rat studied by anterograde transport of horseradish peroxidase conjugated to wheatgerm agglutinin. J Comp Neurol 1988; 268: 91–108.
Jacquin MF, Semba K, Egger MD, Rhoades RW. organization of HRP labeled trigeminal mandibular primary afferent neurons in the rat. J Comp Neurol 1983; 215: 397–420.
Marfurt CF, DelToro DR. Corneal sensory pathway in the rat: A horseradish peroxidse tracing technique. J Comp Neurol 1987; 261: 450–9.
Nagano S, Myers JA, Hall RD. Representation of the cornea in the brain stem of the rat. Exp Neurol 1975; 49: 653–70.
Kerr FWL, Kruger L, Schwassman H, Stern R. Somatotopic organization of mechanoreceptor units in the trigeminal nuclear complex of the macaque. J Comp Neurol 1968; 134: 127–44.
Nord SG. Somatotopic organization in the spinal trigeminal nucleus, the dorsal column nuclei and related structures in the rat. J Comp Neurol 1967; 130: 343–56.
Panneton WM, Burton H. Corneal and periocular representation within the trigeminal sensory complex in the cat studied with transganglionic transport of horseradish peroxidase. J Comp Neurol 1981; 199: 327–44.
Shigenaga Y, Okamoto T, Nishimori T, Suemune S, Nasution I, Chen K, Tsuru A, Yoshida K, Tabuchi M, Tsuru H. Oral and facial representation in the trigeminal principal and rostral spinal nuclei of the cat. J Comp Neurol 1986; 244: 1–18.
Wall PD, Taub A. Four aspects of trigeminal nucleus and a paradox. J Neurophysiol 1962; 25: 110–26.
Torvik A. Afferent connections to the sensory trigeminal nuclei, the nucleus of the solitary tract and adjacent structures. An experimental study in the rat. J Comp Neurol 1956; 106: 51–142.
Nasution ID, Shigenaga Y. Ascending and descending internuclear projections within the trigeminal sensory nuclear complex. Brain Res 1987; 425: 234–47.
Bruce LL, McHaffie JG, Stein BE. The organization of trigeminotectal and trigeminothalamic neurons in rodents: A double labeling study with fluorescent dyes. J Comp Neurol 1987; 262: 315–30.
Huerta MF, Hashikawa T, Gayoso MJ, Harting JK. The trigeminal olivary projection in the cat: Contributions ofindividual subnuclei. J Comp Neurol 1985; 241: 180–90.
Patrick GW, Robinson MH. Collateral projections from trigeminal sensory nuclei to ventrobasal thalamus and cerebellar cortex in rats. J Morphol 1987; 192: 229–236.
Chung JM, Chung K, Wurster RD. Sympathetic preganglionic neurons of the cat spinal cord: horseradish peroxidase study. Brain Res 1975; 91: 126–31.
Petras JM, Cumminmgs JF. Autonomic neurons in the spinal cord of the rhesus monkey: a correlation of the findings of cytoarchitectonics and sympathetectomy. J Comp Neurol 1972; 146: 189–218.
Shimazu T. Nervous control of peripheral metabolism. Acta Physiol Pol 1979; 30: 1–18.
Shimazu T. Central nervous system regulation of liver and adipose tissue metabolism. Diabetologica 1981; 20: 343–56.
Shimazu T. Reciprocal innervation of the liver: Its significance in metabolic controle. In Szabo AJ, ed. Advances in metabolic disorders, Vol. 10 (CNS Regulation of Carbohydrate metabolism). New York: Academic Press, 1983: 355–384.
Shimazu T. Neuronal regulation of hepatic glucose metabolism in mammals. Diabetes/Metabolism Reviews 1987; 3: 185–206.
Opsahl C. A. Sympathetic nervous system in volvement in the lateral hypothalamic lesion syndrome. Am J Physiol 1977; 232: R128–36.
Van der Tuig JG, Knehmans AW, Romsos DR. Reduced sympathetic nervous system activity in rats with ventromedial hypothalamic lesions. Life Sci 1982; 30: 913–20.
Yoshimatsu H, Niijima A, Oomura Y, Yamabe K, Katafuchi T. Effects of hypothalamic lesions on pancreatic autonomic nerve activity in the rat. Brain Res. 1984; 303: 147–52.
Loewy AD, Araujo JC, Kerr FWL. Pupillodilator pathways in the brainstem of the cat: anatomical and electrophysiological indentification of a central autonomic pathway. Brain Res 1973; 60: 65–91.
Budge JL, Waller AV. Action de la partie cervicale du nerf grand sympathique et d'une portion de la moelle epiniere sur la dilatationde la pupille. CR Acad Sci (Paris) 1851; 33: 370–4.
Hodes R, Magoun HW. Pupillary and other responses from stimulation of the frontal cortex and basal telencephalon of the cat. J Comp Neurol 1942; 76: 461–73.
Hodes R, Magoun HW. Autonomic responses to electrical stimulation of the forebrain and midbrain with special reference to the pupil. J Comp Neurol 1942; 76: 169–90.
Jampel RS. Convergence, divergence, pupillary reactions and accomodations of the eye from foradic stimulation of the macaque brain. J Comp Neurol 1960; 115: 371–99.
Gloster J. Influence of the facial nerve on intra-ocular pressure. Br J Ophthalmol 1961; 45: 259–78.
Horst ter G. Descending pathways from the lateral and ventromedial hypothalamic areas to the autonomic centers of medula oblongata and spinal cord. The hypothalamus intrinsic connections and outflow pathways to the pancreas. Groningen (The Netherlands): Stichting C. Regenboog, 1986: 103–27.
Saper CB, Loewy AD, Swanson LW, Cowan WH. Direct hypothalamo-autonomic connection. Brain Res 1976; 117: 305–12.
Rando TA, Bowers CW, Zigmond RE. Localization of neurons in the rat spinal cord which project to the superior cervical ganglion. J Comp Neurol 1981; 196: 73–83.
Langley JN. The sympathetic and other related systems of nerves. In Schafer EA, ed. Textbook of Physiology. Edinbourgh: Pentland, 1900, Vol. 2: 616–96.
Brooks-Fournier R, Coggeshall RE. The ratio of pregangionic cells in the sympathetic nervous system of the rat. J Comp Neurol 1981; 197: 207–16.
Eccles JC. Facilitation and inhibition in the superiocervical ganglion. J Physiol 1935; 85: 207–38.
Dail WG, Barton S. Structure and organization of mammalian sympathetic ganglia: Autonomic ganglia. New York: John Wiley, 1983: 3–26.
Grillo M. Ultrastructural evidence for a sensory innervation of some SIF cells in the rat superior cervical ganglia. Anat Rec 1978; 190: 407.
Duke-Elder S, Wybar RW. System of ophthalmology. St. Louis: Mosby, 1961, Vol II: 813–74.
Warwick R. The ocular parasympathetic nerve supply and its mesencephalic sources. J Anat 1954; 88: 71–93.
Sugimoto T, Itoh K, Mizuno N. Direct projections from the Edinger-Westphal nucleus to the cerebellum and spinal cord in the cat: An HRP study. Neurosci Lett 1978; 9: 17–22.
Loewy AD, Saper CB. Edinger-Westphal nucleus: projection to the brain stem and spinal cord in the cat. Brain Res 1978; 150: 1–27.
Loewy AD, Saper CB, Yamodis ND. Re-evaluation of the efferent projections of the Edinger-Westphal neucleus in the cat. Brain Res 1978; 141: 153–9.
Sugimoto T, Itoh K, Mizuno N. Localization of neurons giving rise to the oculomotor parasympathetic outflow: A HRP study in cats. Neurosci Lett 1977; 7: 301–5.
Kuwayama Y, Grimes PA, Ponte B, Stone RA. Autonomic neurons supplying the rat eye and the intraorbital distribution of vasoactive intestinal peptide (VIP)-like immunore-activity. Exp Eye Res 1987; 44: 907–22.
Ruskell GL. The orbital branches of the pterygopalatine ganglion and their relationship with internal carotid nerve branches in primates. J Anat 1970b; 106: 323–39.
Ruskell GL. Peripheral nerve analysis using Wallerian degeneration: nerves relating to the ciliary ganglion. Exp. Eye Res 1974; 18: 417–8.
Arvidson B. Retrograde transport of horseradish peroxidase in sensory and adrenergic neurons following injection into the anterior eye chamber. J Neurocytol 1979; 8: 751–64.
ten Tusscher MPM, Klooster J, Vrensen GFJM. The innervation of the rabbit's anterior eye segment: A retrograde tracing study. Exp Eye Res 1988; 46: 717–30.
Rambourgh A, Clermont Y, Beaudet A. Ultrastructural features of six types of neurons in rat dorsal root ganglia. J Neurocyt 1983; 21: 47–66.
Prechtl JC, Powley TL. B-afferents: A fundamental division of the nervous system mediating homeostasis? Behav Brain Sci 1990; 13: 289–311.
ten Tusscher MPM, Klooster J, Baljet B, van der Werf F, Vrensen GFJM. Pre- and post-ganglionic nerve fibres of the pterygopalatine ganglion and their allocation to the eyeball of rats. Brain Res 1990; 517: 315–23.
Beckers HJM, Klooster J, Vrensen GFJM, Lamers WPMA. Ultrastructural indentification of trigeminal nerve terminals in the pterygopalatine ganglion of rats: An anterograde tracing and immunohistochemical study. Brain Research 1991; 557: 22–30.
Suzuki N, Hardebo JE, Owman Ch. Trigeminal fibre collaterals storing substance P and calcitoningene-related peptide associate with ganglion cells containing choline acetyltransferase and vasoactive intestinal polypeptide in the sphenopalatine ganglion of the rat. An axon reflex modulating parasympathetic ganglionic activity? Neuroscience 1989; 30: 595–604.
ten Tusscher MPM, Klooster J, van der Want JJL, Lamers WPMA, Vrensen GFJM. The allocation of nerve fibres to the anterior eye segment and peripheral ganglia of rats, II: The sensory innervation. Brain Res 1989; 494: 95–104.
Beckers HJM, Klooster J, Vrensen GFJM, Lamers WPMA. Sympathetic innervation of the rat's eye and peripheral ganglia: an electron microscopic autoradiographic tracing study. Graefe's Arch Clin Exp Ophthalmol, in press.
ten Tusscher MPM, Klooster J, van der Want JJL, Lamers WPMA, Vrensen GFM. The allocation of nerve fibres to the anterior eye segment and peripheral ganglia of rats, II: The sympathetic innervation. Brain Res 1989; 494: 105–13.
Hökfelt T. Distribution of noradrenaline storing particles in peripheral adrenergic neurons as revealed by electron microscopy. Acta Physiol Scand 1969; 76: 427–40.
Chouchkov C, Lazarov N, Davidof M. Serotonoin-like immunoreactivity in the cat trigeminal ganglion. Histochemistry 1988; 88: 637–9.
Hara S, Kobayashi S, Sugita K, Tsukahara S. Innervation of dog ciliary ganglion. Histochemistry 1982; 76: 295–301.
Grimes P, Sallmann von L. Comparative anatomy of the ciliary nerves. Arch Ophthalmol 1960; 64: 81–91.
Tamamaki N, Nojyo Y. Intracranial trajectories of sympathetic nerve fibers originating in the superior cervical ganglion in the rat: WGA-HRP anterograde labeling study. Brain Res 1987; 437: 387–92.
Beckers HJM, Klooster J, Vrensen GFJM, Lamers WPMA. Facial parasympathetic innervation of the rat choroid, lacrimal glands and ciliary ganglion: an ultrastructual pterygopalatine tracing and immunohistochemical study. Ophthal Res, in press.
Hogan MJ, Alvarado JA, Weddell JE. The cornea. Histology of the human eye. Philadelphia: Saunders, 1971: 55–112.
Zander E, Wedell G. Observations on the innervation of the cornea. J Anat 1951; 85: 68–99.
Rozsa AJ, Beuerman W. Density and organization of free nerve endings in the corneal epithelium of the rabbit. Pain 1982; 14: 105–20.
Schimmelpfenning B. Nerve structures in human central corneal epithelium, Graefe's Arch Clin Exp Ophthalmol 1982; 218: 14–20.
Beckers HJM, Klooster J, Vrensen GFJM, Lamers WPMA. Ultrastructural indentification of trigeminal nerve endings in the rat cornea and iris. Investigative Opthalmology & Visual Scienc 1992; 33: 1979–86.
Ehinger B. Ocular and orbital vegetative nerves. Acta Physiol Scand 1966; 67 (suppl.): 1–35.
Laties A, Jacobowitz D. A histochemical study of the adrenergic and cholinergic innervation of the anterior segment of the rabbit eye. Invest Ophthal 1964; 3: 592–600.
Laties A, Jacobowitz D. A comparative study of the autonomic innervation of the eye in monkey, cat and rabbit. Anat Rec 1966; 156: 383–96.
Katz DM, Markey KA, Goldstein M, Black IB. Expression of catecholaminergic characteristics by primary sensory neurons in the normal adult rat. Proc Nat Acad Sci USA 1983; 80: 3526–35.
Katz DM, Adler JE, Black IB. Cathecholaminergic primary sensory neurons: autonomic targets and mechanisms of transmitter regulation. Federation Proc 1987; 46: 24–9.
Marfurt CF. Sympathetic innervation of the rat cornea as demonstrated by the retrograde and anterograde transport of horseradish peroxidase—wheatgerm aggutinin. J Comp Neurol 1988; 268: 147–60.
Morgan C, DeGroat WC, Jannetta PJ. Sympathetic innervation of the cornea from the superior cervical ganglion. An HRP study in the cat. J Auton Nerv System 1987; 20: 179–83.
Fox AE, Powley TL. False-positive artifacts of tracer strategies distort autonomic connectivity maps. Brain Res 1989; 14: 53–77.
ten Tusscher MPM, Klooster J, Vrensen GFJM. Satellite cells as bloodganglion cell barrier in autonomic ganglia. Brain Res 1989; 490: 95–102.
Beckers HJM, Klooster J, Vrensen GFJM, Lamers WPMA. Substance P. in rat corneal and iridal nerves: an ultrastructural immunohistochemical study. Ophthal Res, in press.
Andersson SE. Responses to antidromic trigeminal nerve stimulation, substance P, NKA, CGRP and capsaicin in the rat eye. Acta Physiol Scan 1987; 131: 371–37.
Bill A, Stjernschantz J, Mandahl A, Brodin E, Nilsson G. Substance P: release on trigeminal nerve stimulation, effects in the eye. Acta Physiol Scan 1979; 106: 371–3.
Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience 1988; 24: 739–68.
Oksala O, Stjernschantz J. Effects of calcitonin gene-related peptide in the eye: A study in rabbits and cats. Invest Opthalmol Vis Sci 1988; 29: 1006–11.
Wahlestedt C, Beding B, Ekman R, Oksala O, Sternschantz J, Hakanson R. Calcitonin gene-related peptide in the eye: Release by sensory nerve stimulation and effects associated with neurogenic inflammation. Regul Pept 1986; 16: 107–15.
Stone RA, Laties AM. Neuroanatomy and neuroendocrinology of the chamber angle. In Krieglstein GK, ed. Glaucoma Update III. Berlin: Springer Verlag, 1987: 1–16.
Björklund H, Hökfelt T, Goldstein M, Terenius L, Olson L. Appearance of the noradrenergic markers tyrosine hydroxylase and neuropeptide Y in cholinergic nerves of the iris following sympathectomy. J Neurosci 1985; 5: 1633–43.
Terenghi G, Polak JM, Ghatei MA, Mulderry PK, Butler JM, Unger WG, Bloom SR. Distribution and origin of calcitonin gene-related peptide (CGRP) immunoreactivity in the sensory innervation of the mammalian eye. J Comp Neurol 1985; 233: 506–16.
Zhang SQ, Terenghi G, Unger WG, Ennis KW, Polak J. Changes in substance P- and neuropeptide Y-immunoreactive fibers in rat and guinea-pig irides following unilateral sympathetectomy. Exp Eye Res 1985; 39: 365–72.
Lundberg JM, Terenius L, Hökfelt T, Goldstein M. High levels of neuropeptide Y in peripheral noradrenergic neurons in various mammals including man. Neurosci Lett 1983; 42: 167–72.
Edvinsson L, Ekblad E, Hakanson R, Wahlestedt C. Neuropeptide Y potentiates the effect of various vasoconstrictor agents on rabbit blood vessels. Br J Pharmacol 1984; 83: 519–25.
Ekblad E, Edvinsson L, Wahlestedt C, Uddman R, Hakanson R, Sundler F. Neuropeptide Y co-exists and cooperates with noradrenaline in perivascular nerves. Regul Pept 1984; 8: 225–35.
Piccone M, Littzi J, Krupin T, Stone RA, Davis M, Wax MB. Effects of neuropeptide Y on the isolated rabbit iris dilator muscle. Invest Ophthalmol Vis Sci 1988; 29: 330–2.
Uddman R, Alumets J, Ehinger B, Hakanson R, Loren I, Sundler F. Vasoactive intestinal peptide nerves in ocular and orbital stuctures of the cat. Invest Ophthalmol Vis Sci 1980; 19: 878–85.
Nilsson SFE, Bill A. Vasoactive intestinal polypeptide (VIP): Effects in the eye and on regional blood flows. Acta Physiol Scand 1984; 121: 385–92.
Mittag WT, Tormay A, Podos SM. Vasoactive intestinal peptide and intraocular pressure: Adenylate cyclase activation and binding sites for vasoactive intestinal peptide in membranes of ocular ciliary processes. J Pharmacol Exp Ther 1987; 241: 230–5.
Sears ML. Regulation of aqueous flow by the adenylate cyclase receptor complex in the ciliary epithelium. Am J Ophthalmol 1985; 100: 194–8.
Jumblatt JE, Gooch JM. Neuropeptide Y modulates adenylate cyclase in the rabbit iris, ciliary body and ciliary epithelium. Exp Eye Res 1990; 51: 229–31.
Fredholm BB, Jansen I, Edvinsson L. Neuropeptide Y is a potent inhibitor of cyclic AMP accumulation in feline blood vessels. Acta Physiol Scand 1985; 124: 467–9.
Ohia SE, Jumblatt JE. Effect of neuropeptide Y on norepinepherine release in the rabbit iris-ciliary body. Invest Ophthalmol Vis Sci 1989; 30 (suppl.): 21.
Sears ML, Sherk TE. The trabecular effect of noradrenaline in the rabbit eye. Invest Ophthalmol 1964; 3: 157–63.
Osborne NN, Barnett NL. Calcitonin gene-related polypeptide stimulates C-AMP production in the iris-ciliary body complex. Exp Eye Res 1991; 53: 131–133.
Tower SS. Unit for sensory reception in the cornea with notes on nerve impulses from sclera, iris and lens. J Neurophysiol 1940; 3: 486.
Trzerciakowski JP. Review: Central control of intraocular pressure. J Ocular Pharmacol 1987; 3: 367–378.
Lele PP, Grimes PA. The role of neural mechanisms in the regulation of intraocular pressure in the cat. Exp Neurol 1960; 2: 199–220.
Clark CV, Mapstone R. Autonomic neuropathy in ocular hypertension. The Lancet 1985; 27 July: 185–7.
Barany EH. Transient increase in outflow facility after superior cervical ganglionectomy in rabbits. Arch Ophthal 1962; 67: 303–11.
Canon WB, Rosenbluth A. The supersensitivity of denervated structures: A law of denervation. New York: MacMillan, 1949.
Eakins KE, Eakins HMT. Adrenergic mechanisms and the outflow of aqueous humour from the rabbit eye. J Pharmacol 1964; 144: 60–5.
Greaves DP, Perkins ES. Influence of the sympathetic nervous system on the intraocular pressure and vascular circulation of the eye. Br J Ophthalmol 1952; 36: 258–64.
Langham ME, Taylor CB. The effect of superior cervical ganglionectomy on the intraocular pressure. J Physiol 1959; 147: 58.
Langham ME, Taylor CB. The influence of superior cervical ganglionectomy on intraocular dynamics. Br J Ophthal 1960; 35: 445–58.
Lieb WcA, Guerry D, Ellis LJ. Effects of superior cervical ganglionectomy on aqueous humour dynamicx. Arch Ophthal 1958; 60: 31–5.
Langham ME, Taylor CB. The influence of pre- and postganglionic section of the cervical sympathetic on the intraocular pressure of rabbits and cats. J Physiol 1960; 152: 437–46.
Langham ME, Rosenthal AR. Role of cervical sympathetic nerve in regulating intraocular pressure and circulation. Am J Physiol 1966; 210: 786–94.
Langham M. E. Adrenoreceptor mechanisms in the outflow channels of normal and glaucomatous eyes. In Kriegstein GK, Leydhecker 〈SQUF〉〈SQUF〉〈SQUF〉, eds. Glaucoma Update. Berlin: Springer Verlag, 1978.
Brubaker RF, Gaasterland D. The effect of isoproterenol on aqueous humor formation in humans. Invest Ophthalmol Vis Sci 1984; 25: 357–61.
Gregory DS, Aviado DG, Sears ML. Cervical glanglionectomy alters the circadian rhythm of intraocular pressure in New Zealand White rabbits. Current Eye Res 1985; 4: 1273–9.
Reiss GR, Lee DA, Topper JE, Brubaker RF. Aqueous humour flow during sleep. Invest Ophthal Vis Sci 1984; 25: 776–8.
Erickson-Lamy KA, Kaufman PL. Effect of cholinergic drugs on outflow facility after ciliary ganglionectomy. Invest Ophthalmol Vis Sci 1988; 29: 491–4.
Armaly MF. Studies on intraocular effect of the orbital parasympathetic, II: Effect on the intraocular pressure. Arch Ophthal 1959; 62: 117–24.
Greaves DP, Perkins ES. Influence of the third cranial nerve on intraocular pressure. Br J Ophthalmol 1953; 37: 54–7.
Von Hippel A, Gruunhagen A. Über den Einfluss der Nerven auf die Höhe des intraocularen Druckes. v. Graefe's Arch Ophthalmol 1868; 14: 219–58.
Macri FJ, Cevario SJ. Ciliary ganglion stimulation, I: Effects on aqueous humour inflow and outflow. Invest Ophthal 1975; 14: 28–33.
Greaves DP, Perkins ES. The seventh cranial nerve and intraocular pressure. J Physiol 1956; 134: 393–8.
Bernard C. Leçons sur la physiologie et la pathologie du système nerveux. Paris, 1858, Vol II: 96 and 205.
Hartmann E. La neurotomie retro-gasserienne. Paris: Doin, 1924.
Henderson EE, Starling EH. The influence of changes in the intraocular circulation on the intraocular pressure. J Physiol 1904; 31: 305–19.
Magendie F. De l'influence de la cinquieme paire de nerfs sur la nutrition et les fonctions de l'oeil. J Physiol Exp Pathol 1824; 4: 176–200.
Von Sallmann L, Fuortes MGF, Macri FJ, Grimes P. Study of afferent electric impulses induced by intraocular pressure changes. Am J Ophthalmol 1966; 45: 211–20.
Maurice DM. Constriction of the pupil in the rabbit by antidromic stimulation of the trigeminal nerve. J Physiol 1954; 123: 45.
Perkins ES. Influence of the fifth cranial nerve on the intraocular pressure of the rabbit eye. Br J Ophthal 1957; 41: 257–300.
Stjernschantz J, Geyer C, Bill A. Electrical stimulation of the fifth cranial nerve in rabbits: effects on ocular blood flow, extravascular albumin content and intraocular pressure. Exp Eye Res 1979; 28: 229–38.
Unger WG, Butler JM, Cole DF. Postaglandin and an increased sensitivity of the sympathetically denervated rabbit eye to laser induced irritation of the iris. Exp Eye Res 1981; 32: 699–707.
Gloster J, Greaves DP. Effect of diencephalic stimulation upon intraocular pressure. Br J Ophthalmol 1957; 41: 513–32.
Schmerl E, Steinberg B Seperation of diencephalic centers concerned with pupillary motility and ocular tension. Am J Ophtalmol 1950; 33: 1379–81.
Eakins KE, Ryan SJ. The action of sympathetic amines on the outflow of aqueous humour from the eye. Br J Pharmacol 1964; 23: 374–82.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ten Tusscher, M.P.M., Beckers, H.J.M., Vrensen, G.F.J.M. et al. Peripheral neural circuits regulating IOP?. Doc Ophthalmol 87, 291–313 (1994). https://doi.org/10.1007/BF01203340
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01203340