Abstract
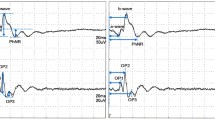
Recent evidence suggests that retinal hypoxia and ischemia affect the standing potential of the eye and the activity of the photoreceptors. To test whether chronically elevated intraocular pressure would produce similar effects, we measured electro-oculograms in two groups of patients: ocular hypertensive patients and patients with primary open-angle glaucoma. There were significant differences among the average electro-oculogram ratios of these groups compared to age-similar controls. The control observers had an average light-peak/dark-trough ratio of 2.86, the ocular hypertensive patients had an average ratio of 2.44, and the patients with primary open-angle glaucoma had an average ratio of 2.07. This indicates that long-term elevations in intraocular pressure can decrease the light peak of the electrooculogram, even in patients with no other evidence of glaucomatous damage. This deficit may have its origins in the sensitivity of the outer retina to choroidal ischemia.
Similar content being viewed by others
Abbreviations
- FOT:
-
fast oscillation trough
- IOP:
-
intraocular pressure
- OHT:
-
ocular hypertension
- POAG:
-
primary open-angle glaucoma
- SP:
-
standing potential
References
Arden GB, Kelsey JH. Changes produced by light in the standing potential of the human eye. J Physiol 1962; 161: 189–204.
Nilsson SEG, Electrophysiology in pigment epithelial changes. Acta Ophthalmol 1985; 63: 22–27.
Niemeyer G. Electrophysiological testing of the function of the vertebrate retina. Pharmacol Ther 1979; 5: 593–97.
Skoog KO. The directly recorded standing potential of the human eye. Acta Ophthalmol 1975; 53: 120–32.
Nilsson SEG, Skoog KO. Covariation of the simultaneously recorded c-wave and the standing potential of the human eye. Acta Ophthalmol 1975; 53: 721–30.
Steinberg RH. Interactions between the retinal pigment epithelium and the neural retina. Doc Ophthalmol 1985; 60: 327–46.
Gallemore RP, Griff ER, Steinberg RH. Evidence in support of a photoreceptoral origin for the ‘light-peak substance’. Invest Ophthalmol Vis Sci 1988; 29: 566–71.
Skrandies W, Baier M. The standing potential of the human eye reflects differences between upper and lower retinal areas. Vision Res 1986; 26: 577–81.
Witkovksy P, Dudek FE, Ripps H. Slow PIII component of the carp electroretinogram. J Gen Physiol 1975; 65: 119–34.
Linsenmeir RA, Steinberg RH. Mechanisms of hypoxic effects on the cat DC electroretinogram. Invest Ophthalmol Vis Sci 1986; 27: 1385–94.
Caprioli J. Correlation of visual function with optic nerve and nerve fiber layer structure in glaucoma. Surv Ophthalmol Supp 1989; 33: 319–30.
Yancey CM, Linsenmeier RA. The electroretinogram and choroidal PO2 in the cat during elevated intraocular pressure. Invest Ophthalmol Vis Sci 1988; 29: 700–7.
Anderson DR, Davis EB. Sensitivities of ocular tissues to acute pressure-induced ischemia. Arch Ophthalmol 1975; 93: 267–74.
Stefansson E, Wilson CA, Schoen T, Kuwabara T. Experimental ischemia induces cell mitosis in the adult rat retina. Invest Opthalmol Vis Sci 1988; 29: 1050–55.
Drummond JC, Rebuck AS. Effect of hypoxia on the corneoretinal potential in man. Ophthalmic Res 1981; 13: 213–23.
Kreienbuhl B, Niemeyer G. Standing potential and c-wave during changes in PO2 and flow in the perfused cat eye. Doc Ophthalmol 1985; 60: 353–60.
Marmor MF, Donovan WJ, Gaba DM. Effects of hypoxia and hyperoxia on the human standing potential. Doc Ophthalmol 1985; 60: 347–52.
Li WWY, Au CYW, Yew DT. Responses of the pigment epithelium and the neuroretina after intraocular pressure increase. Cell Mol Biol 1986; 32: 435–40.
Holopigian K, Seiple W, Mayron C, Koty R, Lorenzo M. Electrophysiological and psychophysical flicker sensitivity in patients with primary open angle glaucoma and ocular hypertension. Invest Ophthalmol Vis Sci 1990; 31: 1863–68.
Riva CE, Sinclair SH, Grunwald JE. Autoregulation of retinal circulation in response to decrease of perfusion pressure. Invest Ophthalmol Vis Sci 1981; 21: 34–38.
Linsenmeier RA, Mines AH, Steinberg RH. Effects of hypoxia and hypercapnia on the light peak and electroretinogram of the cat. Invest Ophthalmol Vis Sci 1983; 22: 37–46.
Linsenmeier RA. Electrophysiological consequences of retinal hypoxia. Graefes Arch Clin Exp Ophthalmol 1990; 228: 143–50.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mehaffey, L., Holopigian, K. & Seiple, W. Electro-oculogram changes in patients with ocular hypertension and primary open-angle glaucoma. Doc Ophthalmol 83, 103–110 (1993). https://doi.org/10.1007/BF01206208
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01206208