Abstract
Aims/hypothesis
Family and twin studies have reported different estimates of the relative contribution of genetic and environmental factors to the quantitative traits glucose tolerance, insulin secretion, and insulin sensitivity. Our aims were to estimate these relative influences in a large sample of twins from the population and to assess the effect of age.
Methods
In this population-based, cross-sectional study we gave an oral glucose tolerance test to 317 women and 290 men who were same-sex healthy twin pairs between 18 to 67 years of age. The genetic, common environmental and individual environmental variance components for fasting and 120-min glucose and for fasting and 30-min insulin as well as the linear effects of age on these components were estimated by multivariate analysis (using the software FISHER).
Results
In women and men the heritability for fasting glucose was 12 and 38%, for 120-min glucose it was 38 and 43%, for fasting insulin it was 54 and 37%, and for 30-min insulin it was 57 and 47%, respectively. Under the assumption of no non-additive genetic effects (no intra- or inter-gene interaction) there was no strong evidence for common environmental effects, barring significant effects for fasting glucose in women. Heritability decreased with age for 120-min glucose in women and fasting insulin in men, whereas it increased for 120-min glucose in men.
Conclusion/interpretation
This study indicates a limited additive genetic influence on the result of an OGTT, possibly with sex-specific age effects, and generally little or no influence of the common environment. Accordingly, there is a considerable individual environmental variation.
Similar content being viewed by others
In family studies varying heritability estimates have been obtained for the quantitative traits glucose tolerance and indices of insulin secretion and insulin sensitivity. Heritability estimates for glucose measures have been in the range between 0 to 39% [1, 2, 3], whereas the estimates for indices of insulin secretion and insulin sensitivity have been somewhat higher, 28 to 65% [4, 5]. Segregation analyses have provided evidence for a major gene effect on both fasting and 1-h insulin [6]. Segregation at a major gene was also suggested for fasting insulin by using commingling analysis [7].
The discrepant heritability estimates from family studies could be due to the limitation that similarity between members of a pedigree can only distinguish between effects of shared genes and shared environment by the use of strong untested assumptions. Furthermore, these 'heritabilities' reflect only the common set of genes influencing glucose and insulin concentrations through the age range considered.
Based on the assumption that twin pairs, regardless of zygosity status, are equally influenced by the shared environment, twin studies are suitable for disentangling the genetic and common environmental effects. The equal age within-pairs and different ages between-pairs make them also suitable for studies of age effects on the genetic and environmental influences.
Previous twin studies of glucose tolerance and insulin secretion and insulin sensitivity have generally been based on samples that were either small, selected, or including subjects with Type 2 diabetes, and the age groups considered have been in the older adult age range. The heritability estimates from these studies were 38 to 50% for fasting glucose [8, 9], 46 to 88% for 1-h to 2-h post-load glucose [10, 11], 14 to 53% for fasting insulin [9, 10], and 47% for 30-min insulin [8]. However, two large studies of non-diabetic female twin pairs have been carried out for fasting insulin, both showing a heritability of about 50% [7, 12]. Using the euglycaemic clamp technique on a very small sample of twins (41 pairs) a heritability of 37% for insulin sensitivity and 55% for insulin secretion was estimated [13].
We have assessed the genetic and environmental influences on fasting and 120-min blood glucose concentrations and on fasting and 30-min insulin concentrations as measured in a large population-based sample of adult female and male twin pairs not suffering from diabetes or cardiovascular disease. Furthermore, we have investigated the relative influence of genes and common environment as a function of age.
Subjects and methods
Subjects
This study is part of a twin study of the metabolic syndrome and related components (GEMINAKAR) for which subjects were identified through the population-based Danish Twin Registry [14]. A mailed questionnaire was sent to a total of 2099 same-sex twin pairs, all alive and living in Denmark according to civil registry records. The questionnaire included information about the exclusion criteria of the study, i.e. pregnancy, breastfeeding, known diabetes or cardiovascular disease. In 465 pairs at least one of the twins did not want to participate, and in 754 twin pairs at least one of the twins did not answer the questionnaire. If one twin partner in a pair did not respond or was not willing to participate, the pair as such was excluded. In 880 pairs both twins answered positively to the questionnaire. Either based on their written replies or on telephone interviews, 116 pairs were excluded due to the above mentioned exclusion criteria to various other diseases or conditions preventing them from completing a bicycle test, which was part of the examination. A remaining group of 386 monozygotic (MZ) and 378 dizygotic (DZ) pairs were willing and able to participate. To get an equal distribution of twin pairs along the age span in the different zygosity groups, sampling from this group was stratified according to age and sex. A total of 621 twin pairs between 18 to 67 years of age underwent an OGTT. To avoid residual confounding of undiagnosed disease, exclusion criteria based on the measured blood glucose concentrations were as follows: if one or both twins in a pair had a fasting blood-glucose concentration greater than 6.7 mmol/l or a 120-min blood glucose concentration greater than 11.1 mmol/l the twin pair as such was excluded. Zygosity of twins was established using nine polymorphic DNA-based microsatellite markers with the PE Applied Biosystems AmpFISTR Profiler Plus Kit (Perkin Elmer, Foster City, Calif., USA). The study was approved by the regional ethics committee and conducted according to the principles of the Helsinki Declaration.
OGTT
Subjects underwent a standardised 75-g OGTT after a 10- to 12-h overnight fast. Capillary blood and peripheral venous blood samples were taken before oral glucose ingestion, and 30 and 120 min later. Capillary blood glucose concentrations were analysed by the glucose dehydrogenase oxidation method. In a blood glucose range of 4 to 10 mmol/l intra-assay coefficient of variation (CV) was 2.0% and inter-assay CV was 2.2%. Serum insulin concentrations were measured using a two-site, two-step, time-resolved immunofluorometric assay (DELFIA, Turku, Finland) [15]. Cross-reactivities with proinsulin, C-peptide and Des(31,32)-split product in the insulin assay were all less than 0.4%. In a serum insulin range of 15 to 70 pmol/l intra-assay CV was 4.2% and inter-assay CV was 4.9%.
As indicators of glucose tolerance, fasting and 120-min glucose concentration values were used. As indicators of peripheral insulin sensitivity and insulin secretion, fasting and 30-min insulin concentration values were used, respectively.
Statistical analysis
Analyses of twin studies assume that intra-pair variance of MZ twins is due to environmental factors and measurement errors, whereas intra-pair variance in DZ twins is additionally augmented by inter-individual variation in genetic factors. The intra-pair variance due to the influence of the environment can be partitioned into environmental factors shared by the twins during intra-uterine life and early childhood (common environmental variance) and environmental factors specific for each twin (individual environmental variance). It is assumed that common environmental factors are shared to a similar extent by MZ and DZ twins. Comparison of the correlation of the trait of interest in MZ twin pairs with that in DZ twin pairs can therefore provide a means of partitioning observed variation into genetic, common environmental and individual environmental variance components. The heritability is the proportional contribution of genetic variance to total (phenotypic) variation in the trait, and expressed by a ratio between 0 and 1. Simultaneous estimation of additive genetic variance, non-additive genetic variance (due to intra-gene interactions, dominance, and to inter-gene interactions, epistasis), and common environmental variance is not possible because of confounding between their effects [16]. In this study, the tendency for DZ correlations to be about half or more than half of the MZ correlations led to the consistent use of a model partitioning the total phenotypic variance (V p ) into additive genetic (V a ), common environmental (V c ), and individual environmental variance (V e ), the latter including variance due to measurement errors. Hence, we have ignored variation due to non-additive effects [17]. However, our results do not rule out such effects.
The covariance between the observations on a pair of twins (Y 1,Y 2) is,
where k=1 is for MZ pairs and k=½ for DZ pairs. For any trait, demonstration of a statistically significant difference in covariance, and hence in correlation between MZ twin pairs and DZ pairs, is consistent with a genetic determination of variation in the trait.
Models were fitted by maximum likelihood assuming the multivariate normality of the observations [16], using the software FISHER (available at: http://www.biomath.medsch.ucla.edu/faculty/klange/software.html) [18]. This allows simultaneous modelling of the mean effects, the variances, covariances, and variance components as functions of measured covariates as well as the selection of parsimonious models by reference to the likelihood ratio criterion [16]. For all traits, an overall mean and a linear regression on age were fitted. Tests of assumptions and outliers were carried out [19, 20]. To yield approximately normal distributions with reasonable size of variances logarithmical transformation and multiplication by 10 were done for all variables. Under the asymptotic-likelihood theory, standard errors were estimated. Statistical significance was defined by a nominal p value of 0.05 or less.
Differences in parameter estimates between the sexes were tested using the estimates and their asymptotic standard errors. The test statistic was the ratio of the difference between the estimates and the standard error of that difference, which is asymptotically distributed as a normal distribution under the null hypothesis that the parameters are the same in male and female subjects.
In addition to the standard partitioning of variation, total variation and (co)variance components were modelled as linear functions of age using FISHER [18, 20]. If t is the age of a particular twin pair, then the model for the (co)variances was,
and
For each of the variance component x (x=a, c, e), the variance is,
The intercept was chosen at the age of the youngest twin pairs in the data (18 years). For the total and each of the three variance components, an age-dependent variance model was selected if: (i) bx was significant and (ii) the maximum likelihood of the model was significantly different (twice difference in log-likelihood >3.84) from a model without age dependence. A linear function is likely to be robust to outliers and is expected to detect an increasing or decreasing trend.
Results
Based on the exclusion criteria for fasting and 120-min blood glucose concentrations, 14 diabetic twin pairs were excluded from the statistical analysis. A complete OGTT was not obtained in 36 of the remaining 607 twin pairs due to either one of the twins being indisposed, problems of phlebotomy, or due to errors in the laboratory. Descriptive data of the 607 remaining pairs showed no differences in means between women and men (Table 1). Except for women's fasting glucose, the differences in MZ and DZ correlations were significant (Table 2). The individual environmental variance component (Ve) accounted for at least 40% of the total phenotypic variance (i.e. the sum of the three variance components) indicating that the influence of the individual environment including measurement error is considerable for all traits (Table 3). There were no statistically significant differences between women and men in additive genetic and common environmental influences.
Tested as linear functions of age, total phenotypic variances of fasting and 120-min glucose values in both sexes and of fasting insulin in men increased with age. For fasting insulin in women and 30-min insulin in both sexes, age dependency was found neither for the total phenotypic variance nor for any of the variance components.
Fasting and 120-min glucose
The heritability estimate for 120-min glucose in women, and for fasting and 120-min glucose in men were about 40% with no evidence of common environmental effects (Table 3). In women, fasting glucose showed only little evidence for additive genetic effect, whereas a common environmental effect of about one third of the total variance was found.
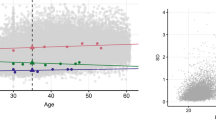
The increase in total phenotypic variance of fasting glucose with age in both sexes was due to increases in all three variance components, but the relative contribution of each variance component did not change with age. For 120-min glucose in women, the increase in total variance with age was due to an increase in the individual environmental component (Fig. 1A), resulting in a decrease in the proportion of genetic variance, i.e. a decrease in the heritability (Fig. 1B). For 120-min glucose in men, the increase in total variance with age was due to an increase in the additive genetic component (Fig. 2A), resulting in an increase in heritability (Fig. 2B).
Age functions of variances and variance proportions for 120-min glucose in women. A Additive genetic variance (bold, solid line), common environmental variance (solid line, close to zero), individual environmental variance (dashed line), and total phenotypic variance (dotted line) as linear functions of age. V a (age)=1.02, V c (age)=0.14, V e (age)=1.09+0.03*(age−18). B The proportion of additive genetic variance (bold, solid line) and common environmental variance (solid line, close to zero) as linear functions of age
Age functions of variances and variance proportions for 120-min glucose in men. A Additive genetic variance (bold, solid line), common environmental variance (solid line, close to zero), individual environmental variance (dashed line), and total phenotypic variance (dotted line) as linear functions of age. V a (age)=0.60+0.05*(age−18), V c (age)=0.17, V e (age)=2.40. B The proportion of additive genetic variance (bold, solid line) and common environmental variance (solid line, close to zero) as linear functions of age
Fasting and 30-min insulin
The heritability for fasting and 30-min insulin was about 55% in women and somewhat lower in men where the heritability for fasting insulin did not reach statistical significance. Furthermore, the increase in total variance with age for fasting insulin in men was due to an increasing influence of the individual environmental component (Fig. 3A), resulting in a decrease in heritability with increasing age (Fig. 3B).
Age functions of variances and variance proportions for fasting insulin in men. A Additive genetic variance (bold, solid line), individual environmental variance (dashed line), and total phenotypic variance (dotted line) as linear functions of age. V a (age)=9.15, V e (age)=7.67+0.33*(age−18). B The proportion of additive genetic variance (bold, solid line) as a linear function of age
Discussion
The influence of genes on the variation in glucose tolerance, insulin secretion, and insulin sensitivity was rather limited. Under the assumption of no non-additive genetic effect there was no strong evidence for common environmental effect except for the effect on women's fasting glucose. Accordingly, there was a considerable individual environmental effect. As measurement error is included in the individual environmental effect, a precise estimate of the influence of environmental factors with putative influence on the development of Type 2 diabetes cannot be obtained.
Even with this twin study being the largest on glucose and insulin measurements from an OGTT in both sexes, it was difficult to obtain precise estimates of variance components. In spite of sex-specific age differences for some of the variance components, differences in parameters between women and men were not detected. Furthermore, large confidence intervals of estimates are observed because precise estimation of variance components (and their ratios) requires much larger samples than, for example, the estimation of fixed effects and covariates. However, the pattern of the present and previous results combined could suggest an interpretation of the actual estimates, which is most likely to reflect the underlying true values. Heritability estimates obtained from analysing data on women and men separately, and particularly the differential changes with age within the two sexes, do suggest that the influences of genes on the responses to the OGTT are not the same in the two sexes and that the genetic influence can change with ageing. For glucose tolerance, men tended to be under more genetic influence than women, with an increasing influence in men and a decreasing influence in women with advancing age. The genetic influence on the indices of insulin secretion and sensitivity appeared more pronounced in women than in men, in whom there, moreover, was a decreasing genetic influence on fasting insulin with age.
However, the interpretation of the significant effects of age on the relative influence of genes, common environment, and individual environment for 120-min glucose in both sexes and for fasting insulin in men is not straightforward. Due to the cross-sectional design, cohort effects and age-specific recruitment bias cannot be ruled out. Especially cohort effect could be of importance in this study comprising subjects born before and after World War II. However, even if there were cohort differences in means and/or variances this might not be reflected in the genetic architecture of the traits, i.e. in the estimates of the genetic contribution to the total variance.
It cannot be excluded that the significant age effects could be caused by departures from the average findings comparing many variables, or on the contrary, that the sample is too small to show all 'true' age effects. The rather large confidence intervals for the variance components could indicate that an even larger sample size could be needed for analysing the effect of age in a cross-sectional study. On the other hand, confident findings of absence of age effects does not exclude that different genes or different environmental exposures could be active at different ages with the same net effect on the phenotype [21].
Our results should be interpreted in the light of the findings for Type 2 diabetes. Type 2 diabetes is considered to be a multifactorial trait [22]. The genetic contribution to Type 2 diabetes is best explained by a polygenic model of inheritance, where the risk for siblings of affected individuals are about 3.5 times the risk for the general population [23]. The wide variation of prevalence among different ethnic groups, e.g. Pima Indians have a prevalence of about 50%, could suggest genetic differences, which is further supported by a decrease in prevalence as genetic admixture with Caucasians increases [24].
On the other hand, apparently inconsistent results have been obtained from studies of concordance rates for diabetes and glucose intolerance in MZ and DZ twins. Most studies have found higher concordance rates for diabetes for MZ than for DZ twins [25, 26, 27]. In contrast, a Danish twin study showed no significant difference between the concordance rates for MZ and DZ twins [8]. However, when glucose intolerance was included in the phenotype, the concordance rate was higher for MZ twins than for DZ twins. Model-fitting analyses estimated a heritability of 26% (CI95: 0–85%) for Type 2 diabetes and a heritability of 61% (CI95: 39–78%) for glucose intolerance [8]. In a Finnish twin study, model-fitting analysis showed a heritability for Type 2 diabetes of 69% in men and 38% in women, who were also under the influence of common environmental factors, accounting for 37% of the total variance [26]. Division of the study sample into four age-by-sex groups showed that in women who were 28 to 59 years of age the concordance rates were zero in both zygosity groups, whereas in men and in women older than 60 the concordance rates were much higher for MZ than for DZ pairs [26]. The findings in these studies suggest that the genetic influence on Type 2 diabetes is less than on glucose intolerance, and that the genetic influence is weaker and the common environmental influence is stronger in pre-menopausal women than in men and post-menopausal women.
Since Type 2 diabetes and glucose intolerance are considered to be the results of a multi-factorial process, it is relevant to question if the genetic and environmental influences are shared between the components of the OGTT response. A study tested 209 twin pairs for pleiotropic effects (two or more phenotypes being under the influence of the same set of genes) on fasting glucose and fasting insulin [9]. They found that the covariance between glucose and insulin could be explained only by individual environmental factors, and therefore, the genetic correlation between glucose and insulin equalled zero. Although no confidence intervals were given for the genetic correlation, it encouraged separate interpretation of the components of the OGTT responses in our study.
Two previous twin studies have estimated a heritability for fasting glucose of 38% and 50%, respectively [8, 9]. In the first study, subjects with Type 2 diabetes were included in the quantitative trait analysis. In the second study, a sample of 209 twin pairs (including opposite-sex pairs) were divided into five sex-by-zygosity groups, and one model was fitted with simultaneous estimation of additive genetic and individual environmental effect without sex difference for fasting glucose and insulin.
Our study on fasting glucose in non-diabetic subjects showed some influence of genes in men and only little influence in the women, most of whom were in the pre-menopausal age range. There was a common environmental influence, which was greater and significant in women. These findings are in agreement with the results of the Finnish twin study of Type 2 diabetes [26]. Taken together, the results suggest a minor role for genes and a role for common environment in women in the pre-menopausal age range, whereas in men there is more influence of genes and less of the common environment. The consistency between the two studies are particularly important in view of the demands of statistical power needed to determined an effect of the common environment [28]. The nature of the common environment that has an effect on women is not known. A twin study found that eating between the main meals was influenced by genetic factors in men, but by common environmental factors in women [29].
In a study of 514 male veteran twin pairs at 42 to 56 years of age, the heritability of post-load glucose (60 min), calculated as h 2=2*(rmz−rdz), with rmz and rdz being the pair-wise correlation for MZ and DZ pairs, respectively, was 88% [11], but it decreased to 52% in 363 of the twin pairs investigated 10 years later [30]. The authors suggested that some of the decrease in heritability could be due to an increase in environmental effects with age. Using the same equation (h 2=2*(rmz−rdz)) on 303 elderly twin pairs, including subjects with Type 2 diabetes, a heritability of 46% and 62% in women and men, respectively, was estimated [10]. We found a heritability of the 120-min post-load glucose concentration at 38% in women and 43% in men. The differences could be due to the use of 60-min and 120-min post-load glucose possibly representing different biological mechanisms, or to differences in ascertainment, age of study subjects, and methods of statistical analyses.
The higher heritability of 120-min post-load glucose compared to fasting glucose in our study suggests that the underlying factors for these two traits are not the same. It has been argued that the 120-min value is more related to obesity [31]. The use of 120-min glucose could thus be more relevant in linkage and association studies searching for genes implicated in the pathogenesis of the classic obesity related Type 2 diabetes. On this background, the introduction of the new diagnostic criteria for Type 2 diabetes by the American Diabetes Association (ADA) [32], which now advocates for using only fasting glucose concentrations, could be problematic.
We have chosen fasting and 30-min insulin as surrogates for insulin sensitivity and insulin secretion, respectively. Others have argued that surrogates that account for the concentration of glucose, i.e. the homeostasis model assessment (HOMA) approach for estimating insulin sensitivity [33, 34], and delta-insulin30–0/delta-glucose30–0 for estimating insulin secretion [35, 36, 36], are better correlated with the clamp technique, which is considered the current "gold standard" [37]. In a previous twin study based on OGTT, the HOMA approach was applied to assess insulin sensitivity in addition to fasting insulin [10]. The results obtained using HOMA did not differ from the results obtained using fasting insulin. As there are several problems in using ratios in analysis of variance [38], we used fasting and 30-min insulin values as indices of insulin sensitivity and secretion, respectively. Furthermore, fasting hyperinsulinaemia itself plays a primary role in the pathogenesis of Type 2 diabetes, independent of insulin resistance [38].
Two large studies of female twins showed heritability estimates for fasting insulin of 48% and 53% [7, 12], respectively, which is in agreement with our results in women. In this study the genetic influence in men was weaker and not statistically significant, but there was no evidence of a common environmental influence. Furthermore, due to an increase in environmental effects, we found a decrease in heritability with age in men. This sex difference suggests that the relative contribution of genes and environment on fasting insulin might not be the same for women and men. Results from same-sex twin pairs cannot reveal to what extent the two sexes are under influence of the same set of genes, but by studying opposite-sex twin pairs, a study did not find evidence for sex-specific genetic effects [9].
The heritability for 30-min insulin of about 50% in both sexes is in accordance with previous twin studies [8, 13].
In conclusion, even with this relatively large sample of twins, it is difficult to obtain precise estimates of variance components and to detect differences in parameters between women and men and changes in parameters with age. Generally, this study suggests rather limited genetic additive influence on fasting glucose and insulin and on post-load glucose and insulin concentrations, and no or little influence of the common environment. There are, however, reasons to suspect sex-specific effects and sex-specific age effects. The modest heritability estimates shown in this study supports the ongoing efforts to prevent Type 2 diabetes by changing the environmental influences, i.e. recommendations about diet, physical exercise and smoking.
Abbreviations
- MZ:
-
Monozygotic
- DZ:
-
dizygotic
- V p :
-
Phenotypic variance
- V a :
-
additive genetic variance
- V c :
-
common environmental variance
- V e :
-
individual environmental variance
- HOMA:
-
homeostasis model assessment
References
Laskarzewski PM, Rao DC, Glueck CJ (1984) The Cincinnati Lipid Research Clinic Family Study: analysis of commingling and family resemblance for fasting blood glucose. Genet Epidemiol 1:341–355
Rice T, Vogler GP, Perry TS, Laskarzewski PM, Province MA, Rao DC (1990) Heterogeneity in the familial aggregation of fasting plasma glucose in five North American populations: the Lipid Research Clinics Family Study. Int J Epidemiol 19:290–296
King H, Rao DC, Bhatia K, Koki G, Collins A, Zimmet P (1989) Family resemblance for glucose tolerance in a Melanesian population, the Tolai. Hum Hered 39:212–217
Sakul H, Pratley R, Cardon L, Ravussin E, Mott D, Bogardus C (1997) Familiality of physical and metabolic characteristics that predict the development of non-insulin-dependent diabetes mellitus in Pima Indians. Am J Hum Genet 60:651–656
Watanabe RM, Valle T, Hauser ER et al. (1999) Familiality of quantitative metabolic traits in Finnish families with non-insulin-dependent diabetes mellitus. Finland-United States Investigation of NIDDM Genetics (FUSION) Study investigators. Hum Hered 49:159–168
Schumacher MC, Hasstedt SJ, Hunt SC, Williams RR, Elbein SC (1992) Major gene effect for insulin levels in familial NIDDM pedigrees. Diabetes 41:416–423
Mayer EJ, Newman B, Austin MA et al. (1996) Genetic and environmental influences on insulin levels and the insulin resistance syndrome: an analysis of women twins. Am J Epidemiol 143:323–332
Poulsen P, Kyvik KO, Vaag A, Beck-Nielsen H (1999) Heritability of type II (non-insulin-dependent) diabetes mellitus and abnormal glucose tolerance—a population-based twin study. Diabetologia 42:139–145
Snieder H, Boomsma DI, Doornen LJ van, Neale MC (1999) Bivariate genetic analysis of fasting insulin and glucose levels. Genet Epidemiol 16:426–446
Poulsen P, Vaag A, Kyvik K, Beck-Nielsen H (2001) Genetic versus environmental aetiology of the metabolic syndrome among male and female twins. Diabetologia 44:537–543
Feinleib M, Garrison RJ, Fabsitz R et al. (1977) The NHLBI twin study of cardiovascular disease risk factors: methodology and summary of results. Am J Epidemiol 106:284–285
Hong Y, Pedersen NL, Brismar K, Hall K, Faire U de (1996) Quantitative genetic analyses of insulin-like growth factor I (IGF-I), IGF-binding protein-1, and insulin levels in middle-aged and elderly twins. J Clin Endocrinol Metab 81:1791–1797
Lehtovirta M, Kaprio J, Forsblom C, Eriksson J, Tuomilehto J, Groop L (2000) Insulin sensitivity and insulin secretion in monozygotic and dizygotic twins. Diabetologia 43:285–293
Kyvik KO, Christensen K, Skytthe A, Harvald B, Holm NV (1996) The Danish Twin Register. Dan Med Bull 43:467–470
Hemmila I, Dakubu S, Mukkala VM, Siitari H, Lovgren T (1984) Europium as a label in time-resolved immunofluorometric assays. Anal Biochem 137:335–343
Lange K, Westlake J, Spence MA (1976) Extensions to pedigree analysis. III. Variance components by the scoring method. Ann Hum Genet 39:485–491
Fisher RA (1918) The correlation between relatives on the supposition of Mendelian inheritance. Trans R Soc (Edinb) 52:399–433
Hopper JL (1988) Review of FISHER. Genet Epidemiol 5:473–476
Hopper JL, Mathews JD (1982) Extensions to multivariate normal models for pedigree analysis. Ann Hum Genet 46:373–383
Hopper JL, Matthews JD (1994) A multivariate model for pedigree analysis and longitudinal data, and the software "FISHER". Aust J Stat 36:153–176
Fabsitz RR, Carmelli D, Hewitt JK (1992) Evidence for independent genetic influences on obesity in middle age. Int J Obes Relat Metab Disord 16:657–666
Lander ES, Schork NJ (1994) Genetic dissection of complex traits. Science 265:2037–2048
Rich SS (1990) Mapping genes in diabetes. Genetic epidemiological perspective. Diabetes 39:1315–1319
Williams RC, Long JC, Hanson RL, Sievers ML, Knowler WC (2000) Individual estimates of European genetic admixture associated with lower body-mass index, plasma glucose, and prevalence of type 2 diabetes in Pima Indians. Am J Hum Genet 66:527–538
Newman B, Selby JV, King MC, Slemenda C, Fabsitz R, Friedman GD (1987) Concordance for type 2 (non-insulin-dependent) diabetes mellitus in male twins. Diabetologia 30:763–768
Kaprio J, Tuomilehto J, Koskenvuo M et al. (1992) Concordance for type 1 (insulin-dependent) and type 2 (non-insulin- dependent) diabetes mellitus in a population-based cohort of twins in Finland. Diabetologia 35:1060–1067
Hopper JL (2000) Why 'common environmental effects' are so uncommon in the literature. In: Spector TD, Snieder H, MacGregor AJ (eds) Advances in twin and sib-pair analysis. Oxford University Press, London, pp 151–165
Castro JM de (1998) Genes and environment have gender-independent influences on the eating and drinking of free-living humans. Physiol Behav 63:385–395
Kalousdian S, Fabsitz R, Havlik R, Christian J, Rosenman R (1987) Heritability of clinical chemistries in an older twin cohort: the NHLBI Twin Study. Genet Epidemiol 4:1–11
Melchionda N, Forlani G, Marchesini G, Baraldi L, Natale S (2002) WHO and ADA criteria for the diagnosis of diabetes mellitus in relation to body mass index. Insulin sensitivity and secretion in resulting subcategories of glucose tolerance. Int J Obes Relat Metab Disord 26:90–96
Gavin JR III, Hughes H, Alberti KGMM et al. (1997) Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 20:1183–1197
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Bonora E, Targher G, Alberiche M et al. (2000) Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 23:57–63
Haffner SM, Miettinen H, Gaskill SP, Stern MP (1996) Decreased insulin action and insulin secretion predict the development of impaired glucose tolerance. Diabetologia 39:1201–1207
Matsumoto K, Miyake S, Yano M (1997) Glucose tolerance, insulin secretion, and insulin sensitivity in nonobese and obese Japanese subjects. Diabetes Care 20:1562–1568
DeFronzo RA, Tobin JD, Andres R (1979) Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 237:E214–E223
Allison DB, Paultre F, Goran MI, Poehlman ET, Heymsfield SB (1995) Statistical considerations regarding the use of ratios to adjust data. Int J Obes Relat Metab Disord 19:644–652
Weyer C, Hanson RL, Tataranni PA, Bogardus C, Pratley RE (2000) A high fasting plasma insulin concentration predicts type 2 diabetes independent of insulin resistance: evidence for a pathogenic role of relative hyperinsulinemia. Diabetes 49:2094–2101
Acknowledgements
The GEMINAKAR project was supported by grants from the Danish Medical Research Fund, The Danish Diabetes Association, The NOVO Foundation, The Danish Heart Foundation, Apotekerfonden, The Foundation of Aage and Johanne Louis-Hansen, The Foundation of Direktør E. Danielsen and Wife, The Foundation of Direktør Kurt Bønnelycke and Wife Grethe, The Foundation of Lægevidenskabens Fremme, The Foundation of Mrs. Asta Florida Bolding, The Foundation of Ove William and Edith Buhl Olesen, The Faculty of Health at University of Southern Denmark, The Danish National Science Foundation, Torkil Steenbeck's Foundation, The Foundation of Gangsted, and King Christian the Tenth's Foundation.
The authors acknowledge Mrs. L.K. Brigsted, Mrs. B. Carstensen, Mr. E. Eilersen, Mrs. E. Beck-Nielsen, Mrs. B. Dalsgaard, and Ms. D. Viborg for their excellent technical assistance. Prof. H. Beck-Nielsen and his staff at Department of Endocrinology, Odense University Hospital, are acknowledged for laboratory analysis of the insulin concentrations. Thanks are deeply expressed to all the twins who took a day off to participate in the GEMINAKAR project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schousboe, K., Visscher, P.M., Henriksen, J.E. et al. Twin study of genetic and environmental influences on glucose tolerance and indices of insulin sensitivity and secretion. Diabetologia 46, 1276–1283 (2003). https://doi.org/10.1007/s00125-003-1165-x
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-003-1165-x