Abstract
Background
To determine whether iodide protects from UVB irradiation-induced destruction of hyaluronate and against UVB injury of cultured human conjunctival fibroblasts.
Methods
Hyaluronate and primary cultured human conjunctival fibroblasts were incubated with various concentrations of iodide and then exposed to UV light irradiation of 312 nm. Hyaluronate destruction was determined by viscosity measurements. Cell viability was assessed with MTT assay.
Results
Iodide protects hyaluronate from UVB light-induced degradation of this macromolecule in a concentration-dependent manner. Incubation of human conjunctival fibroblasts with iodide inhibited cells from damage by UVB light.
Conclusion
Iodide protects hyaluronate, a component of tear fluid and tissues of the anterior part of the eye, against UVB light-induced degradation. Also, injury of human conjunctival cells can be prevented by incubation with iodide before UVB irradiation. The mechanism of protection is likely to include an antioxidative reaction. To support the natural defence mechanisms of the eyes, the administration of an antioxidant such as iodide to artificial tears, for example, may help to prevent the damage of the eye provoked by oxidative stress.
Similar content being viewed by others
Introduction
Dry eye disease is one of the most frequently encountered categories of ocular morbidity. Approximately up to 20% of adults aged 45 years or older report typical symptoms [3]. In the United States the prevalence of mild to moderate dry eye is approximately 10 million [25, 34].
The pathogenesis of dry eye is believed to be multifactorial and can be related to tear deficiency and evaporation [21]. However, the dramatic increase in dry eye may be primarily due to additional factors that have exerted an increasing influence on the population in recent years. Medicines such as antihypertensives, antihistamines, psychotropic drugs and hormonal replacement therapy are known to provoke or intensify the dry eye syndrome [26, 33, 39]. Protracted periods spent working or playing at the computer lead to a reduction of the blinking rate and thus to tear film disorders [42]. Fan heaters, air conditioning and low humidity because of central heating all lead to an increase in the evaporation of tear fluid, thus potentially damaging the anterior part of the eye [20].
The increased air pollution in the course of the past 20 years, coinciding with an increased incidence of dry eye, may also lead to the conclusion that oxidative reactions are directly related to the pathogenesis of dry eyes [1, 46]. The depleted ozone layer is no longer able to act as an effective filter against UV light, allowing intensive UV rays to reach the earth’s surface. Oxidative reactions induced by UV light result in alterations of the eye surface and possibly in DNA changes of the conjunctival and corneal cells, thus leading to their involution, to enzyme alterations and finally to the development of ocular surface disease such as dry eye, pterygium and pingueculae [8, 10, 12, 16, 19, 28, 29]. Highly reactive oxygen species (ROS) destroy components of the tear fluid such as proteins and hyaluronate [18, 35]. Changes in the composition of the tear fluid caused by high-energy ultraviolet light and free radicals affect the interaction of tear fluid components so that the tear film ultimately loses its stability.
The eye protects itself from radical injury by the endogenous antioxidant enzyme systems, such as superoxide dismutase, catalase and glutathione peroxidase [7, 17]. Further antioxidants such as uric acid and ascorbic acid are present in tear fluid [5], and also lactoferrin and lysozyme show antioxidative activities [15, 38, 44]. If the eye is exposed to excessive oxidative stress the scavengers normally present in the tear fluid are apparently no longer capable of preventing damage. Ophthalmic hydrogels used as artificial tear substitutes possess antioxidant properties and tend to reduce reactive oxygen species [9]. To increase this therapeutic effect in ocular surface disorders involving oxidative stress, additional antioxidants might be of interest in the future.
In preparations containing iodide, antioxidative and radical scavenger effects have been observed [31, 45, 47, 48]. Also treatment of the ocular surface with brine containing iodide contributes to an increase of the antioxidant status in tear fluid [32].
The purpose of the present study was to determine whether iodide in different concentrations has a protective effect against UVB irradiation on tear fluid components and cells of the ocular surface.
Material and methods
Hyaluronate solution
Hyaluronate produced by streptococcus equi (Novoselect GmbH, Berlin) was dissolved in physiological saline (0.5 mg/ml 0.9% NaCl), producing a clear viscous solution. To determine the protective effect of potassium iodide (KI) on the destruction of hyaluronate due to UVB light, KI (Merck, Darmstadt, Germany) was added in a concentration of 0.1, 0.5, 2.5 and 5.0 mM to the hyaluronate solution before irradiation.
Irradiation of hyaluronate by UV light
For irradiation, a VL-115-M UV lamp (30 W, wavelength: 312 nm, Vilber Lourmat, Marne La Vallee, France) was used, and radiant energy was measured with an Optometer P 9710 (Gigahertz Optik, Puchheim, Germany). Twenty milliliters of hyaluronate solution was exposed to irradiation in a UV-permeable quartz glass container that was positioned onto the UV source for 2 h. Hyaluronate solution without irradiation served as a control.
Viscosity measurements
After UVB irradiation the kinematic viscosity of the hyaluronate solutions with and without KI treatment was measured in a KPG-Ubbelohde viscometer (Schott u. Gen., Mainz, Germany) and then compared with a control sample. The tests were carried out in a water bath at a temperature of 20°C.
Conjunctival cell cultures
Primary cultures of human conjunctival fibroblasts were used. Small parts of conjunctiva were removed from human eye bank bulbi and cut into fine pieces with scissors and were rinsed in a physiological NaCl solution. The pieces of conjunctiva were placed in 24-well plates (Corning Incorporated, Corning, N.Y.) and covered with medium (80 ml medium Dulbecco’s MEM +20 ml fetal calf serum +1 ml penicillin/streptomycin + 0.8 ml glutamine, GIBCO, Scotland). Once cell growth and uniform confluent cultures were established, fibroblasts were trypsinised and transferred to tissue culture flasks. Fibroblasts between the 3rd and 7th passage were used. Conjunctival fibroblasts were seeded onto 96-well plates (Becton Dickinson, Le Pont De Claix, France) at a densitiy of 50,000 cells/ml and were treated with various concentrations of KI (0.1, 0.5, 2.5 and 5.0 mM). Medium without KI served as a control. The fibroblasts were irradiated with UVB light 48 h later.
Irradiation of the cells with UV light
In order to measure the influence of UV irradiation on the cells, the medium was replaced by 100 µl PBS per well and the fibroblasts were exposed to a UV source. When taking the spectral irradiance readings at 312 nm, the detector of the Optometer P 9710 was placed in the same position as the cell layer surface during UVB exposure. The energy level of incident radiation (30 mJ/cm2 on the cell layer surface) was derived from the intensity 1.4 mW/cm2 and the exposure time of 22 s. After irradiation the PBS was replaced by medium with or without KI, and the cells were incubated in a CO2 incubator for 2 h. Cells without UV light irradiation served as a control.
Cell viability assay
Cell viability was assessed using the colorimetric MTT assay based on the tetrazolium salt MTT ((3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide; Sigma, Germany). MTT is bioreduced in metabolically active cells into a colored formazan product, and absorbance of MTT reaction product was measured at 545 nm using a microplate reader and compared to the control. Only functional mitochondria can convert the MTT solution producing the typical blue-violet enol-product. This assay is an indirect method to assess cell growth and proliferation since the optical density values can be correlated to the number of living cells in culture [24].
Data analysis
The results of the viscosity measurements and the MTT assays are described by using the means ± SD. The Dunnett test was used to compare the values of UV irradiated samples without KI supplement with different concentrations of KI applied before irradiation. A P value <0.05 was considered significant.
Results
Hyaluronate viscosity measurements
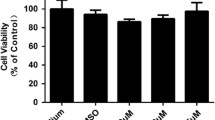
Viscosity measurements were used to determine the damaging influence of UVB light on hyaluronate. Hyaluronate is degraded to fragments of lower molecular weight by irradiation with UVB light as indicated by a decrease of the viscosity of the hyaluronate solution. By adding KI to the hyaluronate solution before irradiation with UVB light, a significant protective effect of KI on the degradation of hyaluronate by UVB light could be observed compared to the hyaluronate solution without KI after irradiation (Fig. 1). An increasing positive influence of KI was seen with doses from 0.1 to 5.0 mM.
Iodide protection of hyaluronate from degradation by UVB irradiation depending on different iodide concentrations: (a) 0.1 mM, (b) 0.5 mM, (c) 2.5 mM and (d) 5.0 mM. Hyaluronate solution served as a control (Hy-control). Addition of KI to the hyaluronate solution did not influence the viscosity (Hy-control+KI). Irradiation with UVB degraded hyaluronate as indicated by the decrease of the viscosity (Hy+UV). Addition of iodide to the hyaluronate solution before irradiation significantly protected hyaluronate from degradation (Hy+KI+UV). Results are means ± SD. Significant differences from UVB-irradiated hyaluronate solution (Hy+UV); *P<0.001
Cell viability assay
In order to examine the influence of UVB light on the viability of human conjunctival fibroblasts, the mitochondrial activity of the cells was monitored by the MTT assay. Addition of KI to the culture medium without UVB irradiation showed no cytotoxicity at concentrations up to 5.0 mM (data not shown). UVB irradiation inhibited the function of mitochondria of the cultured cells. Incubation with KI (0.1, 0.5, 2.5 and 5.0 mM) before irradiation with UVB light showed a protection of the mitochondrial activity in human conjunctival fibroblasts (Table 1). The positive effect of iodide on the reduction of cell viability due to UVB light could be demonstrated up to a concentration of 2.5 mM. Higher doses of iodide did not significantly increase the protective effect as compared to 2.5 mM KI.
Discussion
The results presented here show that iodide can provide a significant protection from UVB-induced degradation of hyaluronate and UVB damage to conjunctival fibroblasts. The mechanism of protection by iodide is likely to include an antioxidative action as discussed by several authors [31, 45, 47, 48].
Due to constant light exposure, the ocular surface is particularly endangered by photochemical reactions [11]. This occurs via either a type I reaction in which an excited irradiated sensitizer in the triplet state directly reacts with a biological substrate by abstracting a proton, or by transferring an electron, or via a type II reaction in which the sensitizer reacts with oxygen, thereby inducing a highly reactive excited singlet state oxygen, or a superoxide ion [40].
Tear fluid consists of a mucous layer, an aqueous layer and a lipid layer. The interaction of all these components produces a stable tear film as needed by the anterior part of the eye. Hyaluronate, a component of the mucous layer of tear fluid and of tissues of the anterior part of the eye [14, 49], is degraded upon irradiation with UV light [18, 35]. In this process, the macromolecule is degraded to fragments of low molecular weight. Hyaluronate is not only destroyed by UV light, a change of the structure of the hyaluronate molecule can also be observed by other oxidative systems such as ozone and cigarette smoke [23, 36].
Also the proteins of tear fluid are destroyed by ozone and UV light, whereby ozone and UV light are especially aggressive in a combined form [36]. Other pollutants containing aggressive components such as cigarette smoke and car exhaust fumes react in a similar way [37].
Since lipids are also extremely sensitive to oxidative reaction [27, 43], all components of tear fluid are degraded by free radicals.
It is conceivable that these environmental factors are damaging where a prior deficiency or instability in tear production exists. However, a primary destruction of tear fluid components could also disrupt the interaction of such components in people with normal tear production, leading to a break up of the tear film and therefore to dry eye conditions.
UVB light also damages the cells of the anterior part of the eye provoking photokeratitis and the occurrence of pterygium and pingueculae [28, 29]. This damage may result from a direct phototoxic reaction in which the absorbed photon energy produces a toxic molecule. Alternatively, after a photosensitized reaction, molecules may absorb energy and then release it to a secondary molecule, which then becomes toxic [40]. Changes to the enzyme pattern and reductions in the concentration of aldehyde and alcohol dehydrogenases [10], increases in acid glycosidases and lysosomal proteases [4] and increases in inflammation mediators after the irradiation of corneal cells with UV light have been observed [19]. Oxygen free radicals have been demonstrated to be cytotoxic for cultured cells, to degrade polysaccharides and DNA, to promote peroxidation of membrane lipids and to alter vascular permeability [13]. In addition, these free radicals play a significant role in the intensification of inflammation because of the generation of chemotactic factors [6].
It is well established that inflammation itself is also associated with a production of free radicals. Activated leukocytes to which the conjunctiva and the cornea are exposed during inflammation are known to produce large amounts of O2 and H2O2 [2], and in the presence of iron the extremely toxic OH. radical can be formed [22].
A marked increase of inflammatory activity and of oxidative reactions was observed in the tear film of patients suffering from ocular surface disorders [1, 41]. In a study of dry eye patients, lipid peroxides and ROS activity were significantly increased in tears when compared to normal persons [1].
The administration of tear substitutes containing substances with radical scavenger activity such as hydrogels may help prevent damage provoked by oxidative stress by supporting the natural defence mechanisms of the eyes [9]. Artificial tear preparations containing a mixture of vitamins A, C and E as well as iodide also confirmed the efficacy of preparations containing free radical scavengers [31].
In the present study we investigated the capability of iodide to protect tear film components and cells of the anterior part of the eye from damage by UVB irradiation.
As shown in our viscosity measurements the destruction of hyaluronate, a glycosaminoglycan present in tears and eye tissues [14, 49], can be prevented by the addition of iodide to the hyaluronate solution before irradiation with UVB light. An increasing positive influence of iodide was seen with doses from 0.1 to 5 mM KI. Similar to the protective effect of iodide on degradation of hyaluronate, addition of iodide to the cell culture led also to a protection of human conjunctival fibroblasts from the negative effect of low dose (30 mJ/ cm2) UVB irradiation [19, 28, 30]. A significant protective effect as shown in the MTT test based on mitochondrial viability was observed at iodide concentrations from 0.5 to 2.5 mM, which did not increase by higher concentrations of 5.0 mM KI.
Although tear fluid and cells may behave differently in vivo, in vitro experiments provide initial information concerning the kind of negative effects by increased environmental pollution. The damage shown to be caused to tear fluid components along with the changes induced in the cells of the anterior part of the eye suggest that increased levels of UVB light may contribute to ocular surface disease. Therefore, in the future, it will be of great importance to develop drugs containing antioxidative components such as iodide ions that could protect the ocular surface from free radical-induced damage.
References
Augustin AJ, Spitznas M, Kaviani N, Meller D, Koch FH, Grus F, Gobbels MJ (1995) Oxidative reactions in the tear fluid of patients suffering from dry eyes. Graefes Arch Clin Exp Ophthalmol 233:694–698
Bradley PP, Priebat DA, Christensen RD, Rothstein G (1982) Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol 78:206–209
Brewitt H, Sistane F (2001) Dry eye disease: the scale of the problem. Surv Opthalmol 45:99–202
Cejkova J, Lojda Z (1995) The damaging effect of UV rays below 320 nm on the rabbit anterior eye segment. II. Enzyme histochemical changes and plasmin activity after prolonged irradiation. Acta Histochem 97:183–188
Choy CKM, Forster Benzie IF, Cho P (2000) Ascorbic acid concentration and total antioxidant activity of human tear fluid measured using the FRASC assay. Invest Ophthalmol Vis Sci 41:3293–3298
Conner EM, Grisham MB (1996) Inflammation, free radicals and antioxidants. Nutrition 12:274–277
Crouch RK, Goletz P, Snyder A, Coles WH (1991) Antioxidant enzymes in human tears. J Ocul Pharmacol 7:253–258
Davies RJH (1995) Ultraviolet radiation damage in DNA. Biochem Soc Trans 23:407–418
Debbasch C, Bruneau De La Salle S, Brignole F, Rat P, Warnet J-M. Baudouin C (2002) Cytoprotective effects of haluronic acid and carbomer 934P in ocular surface epithelial cells. Invest Ophthalmol Vis Sci 43:3409–3415
Downes JE, Swann PG, Holmes RS (1993) Ultraviolet light-induced pathology in the eye: associated changes in ocular aldehyde dehydrogenase and alcohol dehydrogenase activities. Cornea 12:241–248
Elstner EF, Schempp H, Preibisch G, Hippeli S, Oßwald W (1994) Free radicals in the environment, medicine and toxicology. Richelieu Press, London, pp 13–45
Estil S, Olsen WM, Huitfeldt HS, Haaskjold E (1997) UVB-induced formation of (6–4) photoproducts in the rat corneal epithelium. Acta Ophthalmol Scand 75:120–123
Freeman BA, Crapo JD (1982) Free radicals and tissue injury. Lab Invest 47:412–426
Frescura M, Berry M, Corfield A, Carrington S, Easty DL (1994) Evidence of hyluronan in human tears and secretions of conjunctival cultures. Biochem Soc Trans 22:228
Fujihara T, Nagano T, Endo K, Nakamura M, Nakata K (2000) Lactoferrin protects against UV-B irradiation-induced corneal epithelial damage in rats. Cornea 19:207–211
Gallar J, Garia de la Rubia P, Gonzalez GG, Belmonte C (1995) Irriation of the anterior segment of the eye by ultraviolet radiation: influence of nerve blockade and calcium antagonists. Curr Eye Res 14:827–835
Gogia R, Richer SP, Rose RC (1998) Tear fluid content of electrochemically active components including water soluble antioxidants. Curr Eye Res 17:257–263
Horwath J, Schmut O (2000) The influence of environmental factors on the development of dry eye. Contactologica 22:21–29
Kennedy M, Kim KH, Harten B, Brown J, Planck S, Meshul C, Edelhauser H, Rosenbaum JT, Armstrong CA, Ansel JC (1997) Ultraviolet irradiation induces the production of multiple cytokines by human corneal cells. Invest Ophthalmol Vis Sci 38:2483–2491
Korb DR, Greiner JV, Glonek T, Esbah R, Finnemore VM, Whalen AC (1996) Effect of periocular humidity on the tear film lipid layer. Cornea 15:129–134
Lemp MA (1995) Report of the National Eye Institute: industry workshop on clinical trials in dry eyes. CLAO J 21:222–232
McCord JM, Day ED (1978) Superoxide-dependent production of hydroxyl radicals catalysed by iron-EDTA complex. FEBS Lett 86:139
McDevitt CA, Beck GJ, Ciunga MJ, O’Brien J (1989) Cigarette smoke degrades hyaluronic acid. Lung 167:237–245
Mosmann T (1983) Rapid colorimetic assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Moss SE, Klein R, Klein BE (2000) Prevalence of and risk factors for dry eye. Arch Ophthalmol 118:1264–1268
Murube del Castillo J (1991) Das Trockene Auge in Klinik und Praxis. Springer, Berlin Heidelberg New York, pp 3–21
Mustafa MG (1990) Biochemical basis of ozone toxicity. Free Rad Biol Med 9:245–265
Pitts DG, Cullen AP, Hacker PD (1977) Ocular effects of ultraviolet radiation from 295–365 nm. Invest Ophthalmol Vis Sci 16:932–939
Pitts DG, Bergmanson JPG, F Chu LW (1987) Ultrastructural analysis of corneal exposure to UV radiation. Acta Ophthalmol 65:263-273
Podskochy A, Fagerholm P (1998) Cellular response and reactive hyaluronan production in UV-exposed rabbit corneas. Cornea 17:640–645
Rieger G (2001) Anti-oxidative capacity of various artificial tear preparations. Graefe‘s Arch Clin Exp Ophthalmol 239:222–226
Rieger G, Giebenow S, Winkler R, Stoiser E (2000) Der antioxidative Status der Tränenflüssigkeit vor und nach kombinierten Kurbehandlungen in Bad Hall. Spektrum Augenheilkd 14:319–324
Schaumberg DA, Buring JE, Sullivan DA, Dana MR (2001) Hormone replacement therapy and dry eye syndrome. JAMA 286:2114–2119
Schein OD, Munoz B, Tielsch, JM, Bandeen-Roche K, West S (1997) Prevalence of dry eye among the elderly. Am J Ophthalmol 124:723–728
Schmut O, Gruber W, El-Shabrawi Y, Faulborn J (1994) Destruction of human tear proteins by ozone. Free Radic Biol Med 17:164–169
Schmut O, Nassiri Ansari A, Faulborn J (1994) Degradation of hyaluronate by the concerted action of ozone and sunlight. Ophthalmic Res 26:340–343
Schmut O, Faulborn J (1996) Zerstörung menschlicher Tränenproteine durch Autoabgase und Zigarettenrauch. Spektrum Augenheilkd 10:176–178
Shimmura S, Suematsu M, Shimoyama M, Tsubota K, Ocuchi Y, Ishimura Y (1996) Subtreshold UV radiation-induced peroxide formation in cultured corneal epithelial cells: the protective effects of lactoferrin. Exp Eye Res 63:519–526
Slugg A, Ousler GW, Abelson MB (2000) Ocular drying effects of oral antihistamines in the normal population. ARVO Abstract no. 1448
Söderberg PG (1990) Experimental cataract induced by ultraviolet radiation. Acta Ophthalmol 68:10–13
Stern ME, Beuerman RW, Fox Ril, Gao J. Mircheff AK, Pflugfelder SC (1998) The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea 17:584–589
Tsubota K, Nakamori K (1993) Dry eyes and video display terminals. New England J Med 328:584
Vandervliet A, Oneill CA, Eiserich JP, Cross CE (1995) Oxidative damage to extracellular fluids by ozone and possible protective effects of thiols. Arch Biochem Biophys 321:43–50
Van Haeringen NJ (1981) Clinical biochemistry of tears. Surv Opthalmol 26:84–96
Venturi S, Donati FM, Venturi A, Venturi M, Grossi L, Guidi A (2000) Role of iodine in evolution and carcinogenesis of thyroid, breast and stomach. Adv Clin Pathol 4:11–17
Versura P, Profazio V, Cellini M, Torreggiani A, Caramazza R (1999) Eye discomfort and air pollution. Ophthalmologica 213:103–109
Winkler R, Moser M, Buchberger W (1989) Die Wirksamkeit von Jodid als Sauerstoff-Radikalfänger. Wiss Z Humboldt Univ Berl R Med 38:76–79
Winkler R, Moser M (1992) Jodid. Ein potentielles Antioxidans und Sauerstoff-radiakalfänger und seine Rolle bei Peroxidase-Reaktionen. Vitaminspur 7:124–134
Yoshida K, Nitatori T, Uchiyama Y (1996) Localization of glycosaminoglycans and CD44 in the human lacrimal gland. Arch Histol Cytol 59:505–513
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schmut, O., Horwath-Winter, J., Rieger, G. et al. Iodide protection from UVB irradiation-induced degradation of hyaluronate and against UVB-damage of human conjunctival fibroblasts. Graefe's Arch Clin Exp Ophthalmol 242, 279–283 (2004). https://doi.org/10.1007/s00417-003-0829-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-003-0829-z