Abstract
Purpose
To examine the expression of the p27(KIP1), cyclin D1, and proliferating cell nuclear antigen (PCNA) in the retina and retinal pigment epithelium (RPE) after retinal detachment.
Methods
Normal eyes and eyes at 2 or 4 days after retinal detachment with the C57B16 mouse were analyzed by immunocytochemistry using anti-p27(KIP1), anti-cyclin D1, and anti-proliferating cell nuclear antigen (PCNA) antibodies as well as anti-glutamate synthetase (GS) antibody.
Results
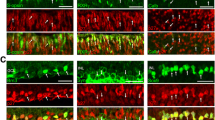
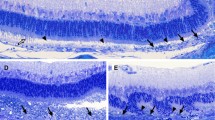
The p27(KIP1) positive nuclei were distributed in the inner nuclear layer (INL) and the RPE of the normal mice eye. In the INL, p27(KIP1) was detected in the middle sublayer, where the nuclei of glutamate synthetase positive Müller cells were situated. In contrast, cyclin D1 was not detected either in the retina or in the RPE. At 2 and 4 days after the retinal detachment, RPE cells under the detached retina were negative for p27(KIP1) and positive for cyclin D1 and PCNA. In the INL of the detached retina, p27(KIP1) was detected after 2 days, but was not detected after 4 days. In contrast, PCNA was not detected in the INL after 2 days, but was detected after 4 days. Cyclin D1 was detected in the middle sublayer of the INL at both 2 and 4 days after the retinal detachment.
Conclusion
These results suggested that degradation of p27(KIP1) and expression of cyclin D1 was involved in the proliferation of the Müller cells as well as RPE cells after retinal detachment.



Similar content being viewed by others
References
Albanese C, Johnson J, Watanabe G, Eklund N, Vu D, Arnold A, Pestell RG (1995) Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem 270:23589–23597
Anderson DH, Stern WH, Fisher SK, Erickson PA, Borgula GA (1981) The onset of pigment epithelial proliferation after retinal detachment. Invest Ophthalmol Vis Sci 21:10–16
Blagosklonny MV, Wu GS, Omura S, el-Deiry WS (1996) Proteasome-dependent regulation of p21WAF1/CIP1 expression. Biochem Biophys Res Commun 227:564–569
Bravo R, Macdonald-Bravo H (1987) Existence of two populations of cyclin/proliferating cell nuclear antigen during the cell cycle: association with DNA replication sites. J Cell Biol 105:1549–1554
Carrano AC, Eytan E, Hershko A, Pagano M (1999) SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat Cell Biol 1:193–199
Fisher SK, Erickson PA, Lewis GP, Anderson DH (1991) Intraretinal proliferation induced by retinal detachment. Invest Ophthalmol Vis Sci 32:1739–1748
Harada T, Imaki J, Hagiwara M, Ohki K, Takamura M, Ohashi T, Matsuda H, Yoshida K (1995) Phosphorylation of CREB in rat retinal cells after focal retinal injury. Exp Eye Res 61:769–772
Harada T, Harada C, Watanabe M, Inoue Y, Sakagawa T, Nakayama N, Sasaki S, Okuyama S, Watase K, Wada K, Tanaka K (1998) Functions of the two glutamate transporters GLAST and GLT-1 in the retina. Proc Natl Acad Sci USA 95:4663–4666
Harper JW, Elledge SJ (1996) Cdk inhibitors in development and cancer. Curr Opin Genet Dev 6:56–64
Hasegawa M (1958) Restitution of the eye after removal of the retina and lens in the newt, Triturus pyrrhogaster. Embryologia 4:1–32
Ikegami Y, Mitsuda S, Araki M (2002) Neural cell differentiation from retinal pigment epithelial cells of the newt: an organ culture model for the urodele retinal regeneration. J Neuröbiol 150:209–220
Jerdan JA, Pepose JS, Michels RG, Hayashi H, de Bustros S, Sebag M, Glaser BM (1989) Proliferative vitreoretinopathy membranes. An immunohistochemical study. Ophthalmology 96:801–810
Kato JY, Matsuoka M, Polyak K, Massague J, Sherr CJ (1994) Cyclic AMP-induced G 1 phase arrest mediated by an inhibitor (p27Kip 1) of cyclin dependent kinase 4 activation. Cell 79:487–496
Kurki P, Vanderlaan M, Dolbeare F, Gray J, Tan EM (1986) Expression of proliferatiog cell nuclear antigen (PCNA)/cyclin during the cell cycle. Exp Cell Res 166:209–219
Machemer R, van Horn D, Aaberg TM (1978) Pigment epithelial proliferation in human retinal detachment with massive periretinal proliferation. Am J Ophthalmol 85:181–191
Machemer R, Aaberg TM, Freeman HM, Irvine AR, Lean JS, Michels RM (1991) An updated classification of retinal detachment with proliferative vitreoretinopathy. Am J Ophthalmol 112:159–165
Moriuchi A, Ido A, Nagata Y, Nagata K, Uto H, Hasuike S, Hori T, Hirono S, Hayashi K, Tsubouchi H (2003) A CRE and the region occupied by a protein induced by growth factors contribute to up-regulation of cyclin D l expression in hepatocytes. Biochem Biophys Res Commun 300:415–421
Ohki K, Yoshida K, Yamakawa A, Harada T, Matsuda H, Imaki J (1995) Jun-b gene expression in rat retinal cells following focal retinal injury. Curr Eye Res 14:1021–1024
Pagano M, Tam SW, Theodoras AM, Beer-Romero P, Del Sal G, Chau V, Yew PR, Draetta GF, Rolfe M (1995) Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science 269:682–685
Polyak K, Lee MH, Erdjument-Bromage H, Koff A, Roberts JM, Tempst P, Massague J (1994) Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 78:59–66
Sherr CJ (1994) G1 phase progression: cycling on cue. Cell 79:551–555
Shirane M, Harumiya Y, Ishida N, Hirai A, Miyamoto C, Hatakeyama S, Nakayama K, Kitagawa M (1999) Down-regulation of p27(Kip l) by two mechanisms, ubiquitin-mediated degradation and proteolytic processing. J Biol Chem 274:13886–13993
Thanos D, Maniatis T (1995) NF-kappa B: a lesson in family values. Cell 80:529–532
Toyoshima H, Hunter T (1994) p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell 78:67–74
Tsvetkov LM, Yeh KH, Lee SJ, Sun H, Zhang H (1999) p27(Kip1) ubiquitination and degradation is regulated by the SCF(Skp2) complex through phosphorylated Thr187 in p27. Curr Biol 19:661–664
Yoshida K, Muraki Y, Ohki K, Harada T, Ohashi T, Matsuda H, Imaki J (1995) C-fos gene expression in rat retinal cells after focal retinal injury. Invest Ophthalmol Vis Sci 36:251–254
Yoshida K, Hu Y, Karin M (2000) IkappaB Kinase alpha is essential for development of the mammalian cornea and conjunctiva. Invest Ophthalmol Vis Sci 41:3665–3669
Yoshida K, Nakayama K, Nagahama H, Harada T, Harada C, Imaki J, Matsuda A, Yamamoto K, Ito M, Ohno S, Nakayama KI (2002) Involvement of p27 (KIP1) degradation by Skp2 in the regulation of proliferation in response to wounding of corneal epithelium. Invest Ophthalmol Vis Sci 43:364–370
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoshida, K., Kase, S., Nakayama, K. et al. Distribution of p27(KIP1), cyclin D1, and proliferating cell nuclear antigen after retinal detachment. Graefe's Arch Clin Exp Ophthalmol 242, 437–441 (2004). https://doi.org/10.1007/s00417-004-0861-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-004-0861-7