Abstract
Purpose
Endogenous endophthalmitis (EE) is a sight-threatening emergency and the aetiology is often multifactorial. Delayed diagnosis may exacerbate the poor visual prognosis. We describe the management and visual outcomes of EE presenting to a tertiary referral centre.
Patients and methods
A prospective consecutive case series of 64 patients presenting with presumed EE from 1997 to 2007 to the Royal Victorian Eye and Ear Hospital were included. All data were collected in a standardized manner. Outcome measures included: visual acuity, microbial profiles, and vitrectomy rate.
Results
In total, 64 cases of EE were identified over the study period with a mean age of 57.5 years, and 53.5% were male. Presenting acuities ranged from Snellen 6/6 to no perception of light (NPL). Identifiable risk factors were present in 78.1%, with the majority related to intravenous drug abuse. A 64.1% culture positivity rate was recorded. A vitrectomy rate of 57, 56, and 21% was recorded in documented bacterial, fungal, and no growth cases, respectively. Final Snellen acuities ranged from 6/6 to NPL. A total of 5 out of 64 eyes were enucleated, of which 3 identified Klebsiellaspecies. Better visual outcome was documented in fungal cases.
Conclusion
EE is a serious ocular condition and has a varied aetiology. Visual outcomes are often poor, irrespective of the method of management. Fungal aetiology often confers a better prognosis, and vitrectomy is advocated for bacterial proven cases.
Similar content being viewed by others
Introduction
Endophthalmitis is a sight-threatening condition defined as any inflammation of the internal ocular spaces. However, in clinical practice it is defined in terms of inflammation secondary to intraocular infection.1 It is classified as either endogenous or exogenous depending on the route of infection. Exogenous endophthalmitis results from direct inoculation as a complication of intraocular surgery, penetrating ocular trauma, intraocular foreign bodies, corneal ulceration, and following a breach of ocular barriers from a periocular infection.2, 3 Following intraocular surgery, specifically, as a complication of cataract surgery, the incidence of endophthalmitis is 0.07–0.32%, and there is a characteristic microbiological spectrum.4
Endogenous endophthalmitis (EE) also termed metastatic endophthalmitis, occurs when organisms disseminate through blood-borne spread and enter the internal ocular spaces through the blood-ocular barrier.5 EE is less common than exogenous endophthalmitis and has been reported to account for 2–8% of all cases of endophthalmitis.1, 6 EE is most often associated with a diagnosed underlying medical condition, including diabetes mellitus, liver disease, cardiac disease, malignancy, in-dwelling catheters, and intravenous drug abuse (IVDU).7 Prompt diagnosis and management is essential if useful vision is to be preserved. Therefore, ophthalmologists, microbiologists, and general physicians need a high level of awareness of this disease.
In most cases, the diagnosis is a clinical one and treatment is initiated empirically while awaiting definitive results from intraocular and/or blood cultures. The role of vitrectomy in EE is controversial, with published studies often referring to the Endophthalmitis Vitrectomy Study visual acuity (VA) thresholds, which may not be applicable to EE, a disease with a very different pathogenesis and natural history to post-surgical endophthalmitis.8, 9, 10, 11 Historically, the visual outcomes following EE have been poor, particularly when the causative organism is identified as one of the Klebsiella species.12, 13
Few large case series have documented the incidence, clinical features, and outcomes of EE. The purpose of this study is to document the incidence, presentation, visual outcome, and microbiological profile of all cases of EE presenting to a tertiary referral centre. The role of vitrectomy in management is outlined.
Materials and methods
One of the authors (PA) maintains a prospective database of all cases of infective endophthalmitis presenting to The Royal Victorian Eye and Ear Hospital, Melbourne, Australia. All cases of EE presenting between 1997 and 2007 were included in this study. Inclusion criteria were: evidence of endophthalmitis in either eye, defined as the presence of anterior/posterior segment inflammation on ophthalmic examination, vitritis or characteristic fundal lesions, and the lack of any temporal relationship to a potential exogenous cause. Collected data included: demographics, predisposing risk factors, clinical examination, investigations performed, microbiological profiles, treatment modalities, and final corrected VA at last follow-up.
All microbiological analysis was carried out at the microbiology laboratory at St Vincents's University Hospital, Melbourne. Confirmed microbiological diagnosis was based on microscopy (Gram-staining) and culture positivity. For microbial analysis, anterior chamber (AC), aqueous samples, and vitreous samples were taken. All samples were obtained in accordance with hospital protocols and under aseptic technique. Aqueous samples were obtained with a 1-ml syringe through an AC paracentesis and vitreous samples were obtained through an aspirated tap through the pars plana or during formal vitrectomy. All samples were processed without delay on culture media selected by the microbiologist based on the likely pathogen. A culture was deemed positive if there was growth of the same organism in more than one media or in which there was a confluence of growth on more than solid media at the inoculation site. The research was conducted in accordance with the Declaration of Helsinki principles and local Ethics Committee guidelines.
Management of all cases consisted of initial biopsy and intravitreal broad spectrum antibiotics or antifungals. Primary vitrectomy was performed, view permitting, in those presenting with visual acuities of perception of light (PL). Intravitreal ceftazidime (2.25 mg/ml) and vancomycin (1.0 mg/ml) were given in all suspected bacterial cases. In suspected fungal cases, based on clinical presentation, intravitreal amphotericin B (5 μg/ml) was also administered. In cases managed with initial intravitreal injection therapy alone, a repeat injection was performed at 48 h if there was no clinical change. Those demonstrating significant improvement were observed. In cases with clinical deterioration, a pars plana vitrectomy was performed, view permitting. All patients were also treated with concomitant systemic intravenous therapy (ciprofloxacin or voriconazole). Topical 1% voriconazole drops were used in suspected fungal cases.
Owing to the disproportionate number of patients presenting with beyond Snellen acuities, (that is, count fingers (CFs), hand movements (HMs), PL, or no perception of light (NPL)), visual results were obtained from the difference in VA pre-treatment and post-treatment and categorized into four groups: (1) improved, (2) no change, (3) deteriorated, and (4) enucleation. An improvement was defined as either a gain of ≥ two lines of Snellen VA, where subjects were within Snellen acuity ranges or for those presenting within the ‘beyond Snellen’ category an improvement of one-measured step or more (for example, from HM to CF, or CF to 6/60)14 (2) no change, and (3) deterioration defined as either a loss of ≥ two lines of Snellen VA, where subjects were within Snellen acuity ranges, or for those following treatment ending up in the ‘beyond Snellen’ a loss of one-measured step or more (for example, from CF to HM, or HM to PL). Fisher's exact test of χ2 was used if any cell frequencies were less than five. All analyses were performed in SPSS version 16.0.1 (SPSS, Chicago, IL, USA) and comparisons were regarded as statistically significant for P≤0.05.
Results
A total of 64 eyes from 64 patients were identified over the 10-year study period. The mean age was 57.5 years (SD 21.3), and 53.8% were male. Presenting Snellen VA ranged from 6/6 to NPL, with a median Snellen VA of 6/60 (Table 1). The mean time from onset of symptoms to presentation was 5.2±9.5 days (range 1–35). There was no association between VA and age or time to presentation.
Of the 64 samples taken from 64 patients, 41 were culture positive (64.1%). There was no growth documented in 23 (35.9%) despite repeat biopsy in initial negative cases (Table 2). Of the culture-positive cases, Gram-positive bacteria were isolated in 6 (14.6%), Gram-negative bacteria in 8 (19.5%), and fungal isolates in 27 (65.9%).
Identifiable risk factors were present in 78.1% of patients. These included: IVDU (38.0%), documented genitourinary infections (12.0%), diabetes mellitus (12.0%), in-dwelling catheters (12.0%), or another miscellaneous identifiable systemic source (26.0%) (Table 3). In culture-positive bacterial isolates, 12 of 14 (85.7%) patients had an identifiable risk factor (Table 3). In culture-positive fungal cases, 21 of 27 (77.8%) patients had a documented risk factor, with 61.9% of those having a positive history of IVDU (Table 3). In culture-negative patients, 17 of 23 patients (73.9%) had a documented risk factor. There was an association between microbial isolate and risk factor for IVDU, central line, diabetes, and liver abscess.
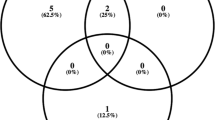
In relation to bacterial cases, 12 of 14 (85.7%) cases presented with ‘beyond Snellen’ VA levels. Pars plana vitrectomy (primary or based on non-response to initial intravitreal antibiotic therapy) was performed in 8 of 14 patients (57.1%). A total of 11 out of 14 patients had no fundal view on presentation, but this did not correlate with vitrectomy. Out of 14 patients, 6 were managed with tap and injection alone. Of these, three (50%) required enucleation despite repeat tap and injection, with rapid clinical deterioration. All enucleated cases isolated Klebsiella species. In the remaining three eyes, one had an improvement in VA (CF to 6/60), one had no change, and one was lost to follow-up. Of those managed with vitrectomy, the VA outcomes were: three (38%) had an improvement in VA (HM to 6/18, HM to 6/36, and 6/24 to 6/9), one had no change, and two deteriorated (both HM to PL) with two patients lost to follow-up. Overall, 3 of 14 (21.4%) had a final VA of ⩾6/18 and 6 of 14 (42.8%) had a final VA of ⩽ PL or required enucleation.
Within the fungal group, 10 of 27 patients presented with ‘beyond Snellen’ VA levels. Pars plana vitrectomy was performed in 15 of 27 (56%) cases. A total of 13 out of 27 (48.1%) patients had a fundal view at presentation. Of those managed with vitrectomy, the VA outcomes were: seven (47%) improved (6/36 to 6/18, 6/48 to 6/9, 6/24 to 6/12, HM to 6/60, CF to 6/18, 6/60 to 6/6, and 6/36 to 6/9), four (27%) had no change and two (13%) deteriorated (6/6 to 6/12, CF to HM), and two were lost to follow-up. Of the nine patients managed with tap and injection alone, seven (78%) improved (6/60 to 6/9, HM to 6/60, 2/60 to 6/12, HM to 2/60, PL to 6/18, HM to 6/60, and 6/36 to 6/6), one had no change, and none deteriorated. The remaining three patients were lost to follow-up. Overall, 13 of 27(48.1%) had a final VA of ⩾6/18 and 2 of 27 (7.4%) had a final VA of ⩽PL or required enucleation.
In 23 patients (35.9%), there was no documented microbial growth. Out of which, 14 (61%) presented with ‘beyond Snellen’ VA. Fundal view was present in 8 of 23 patients at presentation. A vitrectomy rate of 21% was observed among this group. In those managed with vitrectomy, two (40%) improved (CF to 2/60), one remained unchanged, and one deteriorated (6/60 to CF). In those managed with tap and injection alone, four (25%) improved (CF to 6/60, 6/24 to 6/12, HM to 2/60, and 6/18 to 6/9), three (18%) remained unchanged, and one required enucleation. Eight (50%) patients were lost to follow-up from this group (Table 4).
Discussion
EE is a diagnostic and treatment challenge for ophthalmologists. It can occur at any age, and in either sex. It is most commonly associated with an underlying medical condition causing a relative immunosuppressive state such as: IVDU, immune suppression (AIDS, organ transplantation, and diabetes mellitus), post-surgery (most notably tumour procedures), and following invasive diagnostic and dental manipulation. Historically, it has carried a poor prognosis for visual recovery.
In our series, 78.1% of cases had an identifiable risk factor, with the most frequent being IVDU. Fungal isolates accounted for 65.9% of all positive cultures. An association between risk factor and microbiological isolate was found for IVDU and liver abscess only, with Candida and Klebsiella species causally related, respectively. Interestingly, 14 of 64 patients had no identifiable risk factor for disseminated disease despite extensive systemic work-up and investigations. Previous studies, primarily from Asia,9, 10, 15 found liver abscess was the most common infective source in their cohorts with Klebsiella pneumoniae, the most frequent causative organism. Schiedler et al16 demonstrated, in his series from the USA, a more frequent fungal aetiology with 62% of cases positive for Candida albicans. Similarly, Qiong-Yan Zhang et al17 demonstrated a 63% fungal aetiology in their series of EE. The varied spectrum of microbial isolates and the associated risk factors most likely reflects the geographical location of the studies and the patient case-mix presenting to the various institutions. In our study group, the increased numbers of fungal EE cases (65.9% of all culture-positive cases) was causally linked with a history of IVDU and resulted in a statewide public health campaign aimed at reducing the incidence of this condition in this group.18
A culture-positivity rate of 64.1% was obtained in our series agreeing closely with previous reports.1, 3, 19, 20, 21 Delay to diagnosis coupled with institution of systemic poly-pharmacy therapy before presentation represents an added diagnostic challenge in the identification of the offending pathogen in this cohort. Polymerase chain reaction (PCR) is emerging as the diagnostic tool of choice for identifying pathologic microbes in infective endophthalmitis, whether bacterial or fungal. Sowmya et al demonstrated a 100% identification rate using PCR in infectious endophthalmitis in their series. By comparison, utilizing standard sampling and culture techniques, only 37.5% of the isolates were identified among the same cohort.22, 23 Furthermore, more recent studies have shown that real-time PCR has the capability of identifying the offending pathogen in 90 min, offering a potential future rapid and accurate diagnostic tool in this potentially sight-threatening condition.
The treatment of EE should include both ocular and systemic therapy. Systemic intravenous antibiotics or antifungal therapy treat both the ocular disease and the systemic source. Ocular treatment protocols remain unclear. Currently, no clear guidelines on the management of this condition, in particular the role of vitrectomy in its management, exist. Furthermore, the ability to perform safe vitrectomy is often crucial in these highly virulent microorganisms and indeed the presence of significant corneal involvement often precludes this at initial presentation.
In our series, a vitrectomy rate of 57% was observed in bacterial cases, 56% in fungal cases, and 21% in culture-negative cases. In total, five patients in our series required enucleation, none of which had been managed with primary vitrectomy. Three were culture positive for Klebsiella species, one culture positive for Scedosporium prolificans, and one culture negative. It is well-documented that certain specific microbes, particularly Klebsiella carry worse prognosis,12, 13 Our study confirms this, with three of four cases (75%) of culture-positive Klebsiella resulting in enucleation, thus leading to the justification of early aggressive management, including early vitrectomy, view permitting, in these cases. Indeed, Yoon et al10 in their series of Klebsiella-associated EE highlight the importance of a high index of suspicion and early aggressive surgical management in patients with known Klebsiella septicaemia, or those with known hepatic abscess and diabetes mellitus (and thus high risk of Klebsiella), who develop symptoms of EE. Clinical manifestations, with this microbe are often aggressive with exaggerated posterior segment involvement with rapid progression, retinal necrosis, and extensive sub-retinal abscess formation in days. Delayed diagnosis, or initial conservative therapy often leads to poor visual outcome.
VA measurements are difficult to report in this population because of the often beyond Snellen acuities at presentation, making improvement or disimprovement difficult to quantify statistically. Although there does exist possible Snellen conversions for both CF and HM acuity,14 the ability to incorporate improvement from this low baseline, into a series containing better acuities, is problematic. In relation to individual isolates, the VA outcomes in bacterial-proven cases seem to justify early vitrectomy. In bacterial EE cases, 38% of those vitrectomized had improved snellen VA and none required enucleation. However, of those treated with tap and inject alone, only 17% demonstrated improved VA, none had a final VA of >6/60, and 50% required enucleation. Our fungal EE cases appear to be associated with a better prognosis overall, with 56% demonstrating improved VA and 48% having a final VA of ⩾6/18, irrespective of the mode of treatment. Indeed, 7 of 15 (47%) who underwent vitrectomy and 7 of 9 (78%) who did not improved. In the culture-negative cases, VA outcomes were better than confirmed bacterial cases, possibly reflecting the altered virulence of the unidentified microorganism. However, overall, irrespective of causative organism, and similar to previous reports on EE, there were poor visual outcomes in this series.5, 8, 10, 24 Certainly, final VA is often dependent on the vascular inflammatory response, which may be severe and result in long-term sequelae, even in those successfully managed with tap and injection alone. Klebsiella species identification, or demonstration of a liver abscess, in particular, in association with diabetes mellitus, may necessitate early vitrectomy, as demonstrated in this series and previous studies because of the aggressive nature of this microbe and poor outcomes,10, 12, 13 however, it remains unclear whether early vitrectomy would salvage these eyes.
This study has certain limitations. Although the extended prospective follow-up represents a distinct advantage, not all cases were managed within defined protocols. Although an accepted protocol for exogenous bacterial endophthalmitis exists, the management of endogenous disease, particularly fungal disease, does not. In this series, irrespective of method of management, fungal-positive cases seemed to have a favourable visual outcome, perhaps justifying a more conservative approach, at least initially, in its management. Increased numbers with our expanding database will attempt to answer this complex question coupled with a more standardized approach to its management in fungal proven cases.
In conclusion, EE is a difficult disease to diagnose clinically and because of co-morbid conditions it often presents late. Much remains unclear regarding the correct ophthalmic approach particularly in relation to early surgical interventions. However, our data suggests that aggressive early treatment with early vitrectomy in suspected bacterial EE cases and a more conservative approach in suspected fungal cases may be appropriate. However, future studies will be needed to ascertain the role of primary vitrectomy in the management of these complex cases.

References
Chee SP, Jap A . Endogenous endophthalmitis. Curr Opin Ophthalmol 2001; 12: 464–470.
Fan JC, Niederer RL, von Lany H, Polkinghorne PJ . Infectious endophthalmitis: clinical features, management and visual outcomes. Clin Experiment Ophthalmol 2008; 36: 631–636.
Hassan IJ, MacGowan AP, Cook SD . Endophthalmitis at the Bristol Eye Hospital: an 11-year review of 47 patients. J Hosp Infect 1992; 22: 271–278.
Wong TY, Chee SP . The epidemiology of acute endophthalmitis after cataract surgery in an Asian population. Ophthalmology 2004; 111: 699–705.
Wong JS, Chan TK, Lee HM, Chee SP . Endogenous bacterial endophthalmitis: an East Asian experience and a reappraisal of a severe ocular affliction. Ophthalmology 2000; 107: 1483–1491.
Shrader SK, Band JD, Lauter CB, Murphy P . The clinical spectrum of endophthalmitis: incidence, predisposing factors, and features influencing outcome. J Infect Dis 1990; 162: 115–120.
Okada AA, Johnson RP, Liles WC, D’Amico DJ, Baker AS . Endogenous bacterial endophthalmitis. Report of a ten-year retrospective study. Ophthalmology 1994; 101: 832–838.
Jackson TL, Eykyn SJ, Graham EM, Stanford MR . Endogenous bacterial endophthalmitis: a 17-year prospective series and review of 267 reported cases. Surv Ophthalmol 2003; 48: 403–423.
Zhang YQ, Wang WJ . Treatment outcomes after pars plana vitrectomy for endogenous endophthalmitis. Retina 2005; 25: 746–750.
Yoon YH, Lee SU, Sohn JH, Lee SE . Result of early vitrectomy for endogenous Klebsiella pneumoniae endophthalmitis. Retina 2003; 23: 366–370.
Shen X, Xu G . Vitrectomy for endogenous fungal endophthalmitis. Ocul Immunol Inflamm 2009; 17: 148–152.
Scott IU, Matharoo N, Flynn Jr HW, Miller D . Endophthalmitis caused by Klebsiella species. Am J Ophthalmol 2004; 138: 662–663.
Sng CC, Jap A, Chan YH, Chee SP . Risk factors for endogenous Klebsiella endophthalmitis in patients with Klebsiella bacteraemia: a case-control study. Br J Ophthalmol 2008; 92: 673–677.
Holladay JT . Proper method for calculating average visual acuity. J Refract Surg 1997; 13: 388–391.
Keswani T, Ahuja V, Changulani M . Evaluation of outcome of various treatment methods for endogenous endophthalmitis. Indian J Med Sci 2006; 60: 454–460.
Schiedler V, Scott IU, Flynn Jr HW, Davis JL, Benz MS, Miller D . Culture-proven endogenous endophthalmitis: clinical features and visual acuity outcomes. Am J Ophthalmol 2004; 137: 725–731.
Yan H, Chen S, Zhang JK, Yu JG, Han JD . Treatment of postoperative endophthalmitis following cataract surgery without intraocular lens removal. Zhonghua Yan Ke Za Zhi 2009; 45: 684–687.
Aboltins CA, Allen P, Daffy JR . Fungal endophthalmitis in intravenous drug users injecting buprenorphine contaminated with oral Candida species. Med J Aust 2005; 182: 427.
Binder MI, Chua J, Kaiser PK, Procop GW, Isada CM . Endogenous endophthalmitis: an 18-year review of culture-positive cases at a tertiary care center. Medicine (Baltimore) 2003; 82: 97–105.
Chakrabarti A, Shivaprakash MR, Singh R, Tarai B, George VK, Fomda BA et al. Fungal endophthalmitis: fourteen years′ experience from a center in India. Retina 2008; 28: 1400–1407.
Essman TF, Flynn Jr HW, Smiddy WE, Brod RD, Murray TG, Davis JL et al. Treatment outcomes in a 10-year study of endogenous fungal endophthalmitis. Ophthalmic Surg Lasers 1997; 28: 185–194.
Sowmya P, Madhavan HN . Diagnostic utility of polymerase chain reaction on intraocular specimens to establish the etiology of infectious endophthalmitis. Eur J Ophthalmol 2009; 19: 812–817.
Sowmya P, Madhavan HN, Therese KL . Evaluation of three polymerase chain reaction tests targeting morphological transforming region II, UL-83 gene and glycoprotein O gene for the detection of human cytomegalovirus genome in clinical specimens of immunocompromised patients in Chennai, India. Virol J 2006; 3: 20.
Williams MA, McMullan R, Hedderwick S, Mulholland DA, Best RM . Diagnosis and treatment of endogenous fungal endophthalmitis. Ophthalmologica 2006; 220: 134–136.
Acknowledgements
The Gerard Crock Research Fellowship and The Centre for Eye Research, Australia receives operational infrastructure support from the Victorian Government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Connell, P., O'Neill, E., Fabinyi, D. et al. Endogenous endophthalmitis: 10-year experience at a tertiary referral centre. Eye 25, 66–72 (2011). https://doi.org/10.1038/eye.2010.145
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2010.145
Keywords
This article is cited by
-
Literature- and Experience-Based Consensus for Acute Post-operative Endophthalmitis and Endogenous Endophthalmitis in Taiwan
Ophthalmology and Therapy (2024)
-
Recurrent, bilateral endogenous Candida endophthalmitis with multiple focal chorioretinal lesions: management with pars plana vitrectomy and focal endolaser
Journal of Ophthalmic Inflammation and Infection (2022)
-
The microbiological spectrum, antimicrobial resistance pattern, and visual outcomes of endogenous endophthalmitis in West Virginia 2009–2019
International Ophthalmology (2022)
-
Clinical characteristics of endogenous Klebsiella pneumoniae endophthalmitis: a 13-year experience
International Ophthalmology (2022)
-
Combined endophthalmitis and orbital cellulitis in patients with corona virus disease (COVID-19)
Journal of Ophthalmic Inflammation and Infection (2021)