Abstract
Aims
The aim of this study is to evaluate the effect of standard-fluence verteporfin photodynamic therapy (PDT) delivered on the first day of a ranibizumab regimen for choroidal neovascularisation secondary to age-related macular degeneration compared with ranibizumab monotherapy.
Methods
Patients were randomised to sham or standard-fluence verteporfin PDT at baseline. The first of three monthly loading doses of ranibizumab was given on the same day, and thereafter patients received monthly treatment with ranibizumab as required. All patients underwent monthly visual acuity and OCT assessment and 3-monthly fluorescein angiography with follow-up to 1 year.
Results
In all, 18 patients were recruited. The PDT group gained a mean of 2.2 ETDRS letters at 1 year and the sham group gained a mean of 4.4 letters (P=0.47). Both groups required a mean of 1.3 injections of ranibizumab following the 3-month loading phase. Fluorescein angiography at 1 month demonstrated marked choroidal hypoperfusion in all patients treated with PDT with reduced choroidal perfusion persisting to month 12. This did not occur in the sham group.
Conclusion
The addition of standard-fluence verteporfin PDT at baseline to a ranibizumab regimen conferred no benefit in terms of visual acuity or number of ranibizumab injections required at 1 year. The combination of these treatments resulted in persistent reduced choroidal perfusion, which raises potential safety concerns.
Similar content being viewed by others
Introduction
Anti-vascular endothelial growth factor (anti-VEGF) monotherapy is now established as the standard of care for subfoveal choroidal neovascularisation (CNV) secondary to age-related macular degeneration (AMD).1 Although anti-VEGF therapies inhibit growth and permeability of new vessels and help prevent early CNV development, multiple retreatments are needed in most cases, and established CNV may be resistant to VEGF inhibition.2 Photodynamic therapy (PDT) with verteporfin has been shown to occlude established CNV, but causes increased VEGF expression, which may lead to CNV recurrence.3 A combination of these two treatments could therefore potentially decrease the number of anti-VEGF treatments required to control CNV.
The first study to investigate the combination of PDT and ranibizumab, the FOCUS study,4 raised safety concerns related to ocular inflammation, which was reduced following a protocol amendment changing the ranibizumab formulation. Subsequently, the PROTECT study5 has demonstrated that same-day administration of PDT and 0.5 mg liquid ranibizumab is not associated with severe visual loss or ocular inflammation. Uncontrolled trials3 and a large-scale analysis of registry data6 have also suggested that combined therapy has the potential to reduce the number of anti-VEGF treatments required to control neovascular leakage.
Materials and methods
This single-centre, randomised pilot study compared intravitreal injection of ranibizumab and sham PDT treatment to a combination of intravitreal ranibizumab and standard-fluence verteporfin PDT delivered on the first visit. All patients received a further two monthly ranibizumab treatments, and were then retreated as required. The proportion of patients gaining at least 15 letters of ETDRS best-corrected visual acuity (BCVA) at 12 months compared with baseline was assessed as were the number of ranibizumab treatments at 6 and 12 months, BCVA after the initial loading phase of three monthly treatments, the proportion of patients gaining at least 10 letters at 12 months, the proportion of patients losing <10 and 15 letters at 12 months, and time to retreatment after the initial loading phase.
The study received local ethics committee and research and development approval. All patients gave written, informed consent before enrolment. (Clinical trial reference: ISRCTN42639823.)
Patient selection and entry evaluations
A screening visit comprised ocular examination, vision testing, medical history, OCT, and fluorescein angiography assessment. The full inclusion and exclusion criteria are shown in Table 1. The principal criteria were a visual acuity in the study eye of between 24 and 73 ETDRS letters, and an initial fluorescein angiogram demonstrating CNV secondary to AMD with evidence that the CNV extended under the geometric centre of the foveal avascular zone.
CNV were classified as either 100% classic/predominantly classic or minimally classic/occult at baseline to allow stratification before randomisation. For patients with occult with no classic CNV, clinical or angiographic evidence of recent disease progression was required for inclusion (Table 1). Patients who had previously received any other treatment for neovascular AMD were excluded.
Visual assessment
BCVA was measured in both eyes after refraction at baseline and at all follow-up visits using a standardised protocol by certified examiners using modified ETDRS charts. Contrast sensitivity and reading speed were assessed using Pelli-Robson and Bailey-Lovie methods at three monthly intervals.
Imaging
Fluorescein angiography was performed at baseline and repeated at months 1, 3, 6, 9, and 12. The fluorescein angiographic features at baseline and follow-up were determined by two masked assessors (consultant ophthalmologists). OCT examinations were performed at every visit using the STRATUS OCT (Zeiss Meditech, Jena, Germany).
Treatment protocol
Patients were randomised 1 : 1 to receive initial standard-fluence PDT or sham PDT. For the PDT group, the verteporfin infusion was covered by an opaque bag and given over 10 min at a dose of 6 mg/m2. PDT was given at a light dose of 50 J/m2 and a fluence of 600 mW/m2. For the sham group, the procedure was identical, but normal saline was substituted for verteporfin. Both the patient and the treating doctor were masked to the treatment given.
Intravitreal ranibizumab (0.5 mg) was administered on the same day according to the Royal College of Ophthalmologists guidelines,7 and patients self-administered a topical antibiotic (choramphenicol 0.5%) four times a day for 3 days following treatment. Monthly assessment comprised visual acuity, OCT, and full ocular examination. All patients received further ranibizumab treatments at months 2 and 3. Thereafter, ranibizumab was administered if there was a loss of more than 5 letters of BCVA associated with intraretinal or subretinal fluid on OCT, or a >100 μm increase in the mean central 1mm retinal thickness, when compared to the measurement obtained following the three initial ranibizumab loading doses.
The incidence and severity of any ocular adverse events were documented. Treatment was withheld if a loss of BCVA of >30 letters, endophthalmitis, retinal detachment, retinal tear, or vitreous haemorrhage occurred within 14 days of treatment. Treatment was also withheld if intraocular surgery had been performed within 28 days, or there was any evidence of local or systemic infection. An intraocular pressure of >45 mm Hg at 1 h after injection was considered a severe adverse event.
Statistics
As this was a pilot study, there was insufficient evidence to precisely determine sample size in terms of visual acuity outcomes. The sample size was sufficient to give 80% power to detect a reduction in retreatments of two or more at 12-month follow-up. Statistical analysis was performed using SPSS for windows (http://www.spss.com, IBM headquarters, Chicago, IL, USA).
Results
Altogether, 18 patients met the study eligibility criteria and were randomised. All patients completed the study, and no adverse events were recorded. Baseline characteristics and results are shown in Table 2.
Visual acuity
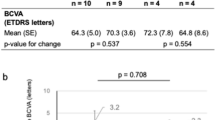
The PDT group gained a mean of 2.2 letters and the sham group 4.4 letters at 1 year (Table 2). This difference was not statistically significant (P=0.47).
The PDT group gained a mean of 3.1 letters and the sham group 6.5 letters after the initial loading phase of the trial (month 4). After 1 month, five of the nine patients in the PDT group lost visual acuity, with three losing between 5 and 15 letters. In the sham group, two of nine patients lost visual acuity 1 month after the first treatment, but no patients lost more than 5 letters. Mean visual acuity for the two groups over the year is shown in Figure 1.
OCT and fluorescein angiography
After 1 year, the mean central retinal thickness was reduced by 138 μ in the PDT group and 103 μ in the sham group (P=0.57). A trend towards a greater reduction after first treatment in the PDT group was seen (Figure 2). All fluorescein angiograms in the PDT group and none of the sham group showed a circle of choroidal hypoflourescence corresponding to the area of PDT treatment at 1 month after initial treatment, and choroidal hypoperfusion persisted throughout the entire 12 months of the study (Figure 3).
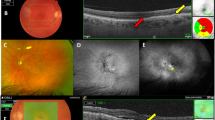
Serial fluorescein angiograms and OCT scans for one patient undergoing combination treatment. Images are from baseline and months 1, 3, 6, 9, and 12. Note the marked choroidal hypoperfusion at 1 month, with abnormal choroidal perfusion persisting over the 12-month follow-up. All combination-treated patients and none of the monotherapy group showed similar choroidal perfusion changes.
Treatment
After the initial 3-month loading phase, both groups required a mean of 1.3 retreatments with ranibizumab over the 12 months of the trial. However, the mean time before the first retreatment was required was 4.6 months in the PDT group and 2.6 months in the sham group.
Discussion
Initial same-day PDT treatment conferred no benefit when compared with treatment with ranibizumab alone in terms of visual acuity, contrast sensitivity, or number of treatments required over the 1-year follow-up period. The initial increase in visual acuity during the first 3-month loading phase of ranibizumab treatment was blunted in the PDT group. The combination group had a lower mean reading score throughout the study and also had a very small (statistically insignificant) improvement in comparison to the monotherapy group. Reading speed is affected by different factors, including visual acuity and concentration levels.
In contrast to our findings, Pruente et al,8 using a similar study protocol but with a 0.3 mg ranibizumab dosage, did demonstrate a reduced need for retreatment with ranibizumab in the group receiving PDT. However, this study also showed no difference between ranibizumab alone and combined therapy at 1 year in terms of visual acuity and a less rapid visual acuity gain in the combined treatment group. This trend towards reduction of the initial effect of ranibizumab when combined with PDT was also seen in the FOCUS study. FOCUS4 is the largest trial of combined therapy to date, and mean visual acuity was lower in the group treated with PDT and ranibizumab than in the PDT-only group at 1 week and 1 month, but favoured the combination-treated group after month 3. However, unlike our study, FOCUS compared PDT only with combined PDT and ranibizumab.
In the combined treatment group of our study, striking choroidal hypoflourescence was observed on fluorescein angiography, and this persisted during the 12 months of follow-up, with recurrence of CNV occurring within this area of reduced choroidal perfusion. Reduced choroidal perfusion is a known consequence of PDT therapy as treatment temporarily closes small- and medium-sized choroidal vessels as well as CNV.9 Concurrent treatment with both steroids10 and ranibizumab11 prolong reduced choroidal perfusion. The PROTECT study11 demonstrated reduced choroidal perfusion using indocyanine green angiography that persisted over 9 months of follow-up in 10 of 11 patients. The choroidal circulation did gradually reperfuse to some degree during this time, and CNV recurred only after reperfusion of the surrounding choriocapillaris. Microperimetry demonstrated improved retinal sensitivity despite reduced choroidal perfusion. No patients suffered severe visual loss in PROTECT and, in contrast to our findings, the mean visual acuity increased by 4.5 letters 1 month after initial treatment. It was concluded that the observed reduced choroidal perfusion did not appear to have any short-term functional significance. However, unlike our study, the PROTECT study was a small case series to evaluate the safety of same-day PDT and intravitreal ranibizumab and did not randomise patients to compare this regime with ranibizumab monotherapy. Furthermore, repeat PDT was administered if there was significant leakage found on fluorescein angiography at months 3, 6, and 9, and ranibizumab was only administered at baseline and at months 1, 2, and 3.
It is not yet known whether inhibiting the process of vascular remodelling has any longer-term effects on the retinal pigment epithelium and retina.12 However, one study reporting 24-month data comparing PDT treatment with PDT and intravitreal triamcinolone11 demonstrated better visual acuity for the combination group in the first year, but progressive visual decline during the second year of follow-up. Reduced choroidal perfusion was still seen on angiography in the combined group at 24 months. Furthermore, the reduction of vision in the steroid-treated group was associated with atrophic chorioretinal and retinal pigment epithelium changes as demonstrated by retinal thinning on OCT and reduced macular fundus autofluorescence when compared with the PDT-only group. If ranibizumab is reducing choroidal reperfusion in a similar manner, it is at least possible that any potential benefit in terms of reduced treatment requirement could be at the cost of longer-term chorioretinal atrophy.
At present there is a lack of solid evidence that combination therapy is superior to ranibizumab monotherapy and, given concerns over potential adverse effects, longer-term results from larger randomised controlled trials are awaited. Strategies to limit reduced choroidal perfusion in combination therapy, such as reduced-fluence PDT with ranibizumab, are currently under investigation.

References
Mitchell P, Korobelnik J-F, Lanzetta P, Holz FG, Pruente C, Schmidt-Erfurth UM et al. Ranibizumab (Lucentis) in neovascular age-related macular degeneration: evidence from clinical trials. Br J Ophthalmol 2010; 94 (1): 2–13. Published Online First: 20 May 2009, doi:10.1136/bjo.2009.159160.
Spaide RF . Rationale for combination therapy in age-related macular degeneration. Retina 2009; 29 (6 Suppl): S5–S7.
Shah GV, Sang DN, Hughes MS . Verteporfin combination regimes in the treatment of neovascular age-related macular degeneration. Retina 2009; 29: 133–148.
Heier JS, Boyer DS, Ciulla TA, Ferrone PJ, Jumper JM, Gentile RC et al. Ranibizumab combined with verteporfin photodynamic therapy in neovascular age-related macular degeneration: year 1 results of the FOCUS Study. Arch Ophthalmol 2006; 124: 1532–1542.
Schmidt-Erfurth U, Wolf S, PROTECT Study Group. Same-Day administration of verteporfin and ranibizumab 0.5 mg in patients with choroidal neovascularisation due to age-related macular degeneration. Br J Ophthalmol 2008; 92: 1628–1635.
Kaiser PK, Registry of Visudyne AMD writing committe. Verteporfin photodynamic therapy combined with intravitreal bevacizumab for neovascular age-related macular degeneration. Ophthalmology 2009; 116 (4): 747–755.
Guidelines for Intravitreal Injections Procedure 2009. http://www.rcophth.ac.uk/about/publications/. (accessed on 21 May 2010).
Pruente C, Hatz K, Henrich PB, Braun B, Sacu S, Schneider U . A randomised double-masked study comparing lucentis monotherapy and pdt combined with lucentis therapy in patients with exudative amd: one year results for bcva and retreatment frequency. Presented at ARVO May 2009, abstract athttp://arvo.abstractsonline.com/plan/SSResults.aspx. (accessed on 13 September 2009).
Schmidt-Erfurth UM, Michels S . Changes in confocal indocyanine green angiography through two years after photodynamic therapy with verteporfin. Ophthalmology 2003; 110: 1306–1314.
Piermarocchi S, Sartore M, Lo Giudice G, Maritan V, Midena E, Segato T . Combination of photodynamic therapy and intraocular triamcinolone for exudative age-related macular degeneration and long-term chorioretinal macular atrophy. Arch Ophthalmol 2008; 126 (10): 1367–1374.
Kiss CG, Simader C, Michels S, Schmidt-Erfurth U . Combination of verteporfin photodynamic therapy and ranibizumab: effects on retinal anatomy, choroidal perfusion and visual function in the PROTECT study. Br J Ophthalmol 2008; 92: 1620–1627.
Schmidt-Erfurth U, Kiss C, Sacu S . The role of choroidal hypoperfusion associated with photodynamic therapy in neovascular age-related macular degeneration and the consequences for combination strategies. Prog Retin Eye Res 2009; 28: 145–154.
Acknowledgements
We acknowledge Ms S Patra for her contribution as one of the assessors of fluorescein angiograms in this study and we also like to acknowledge the dedication of all the Clinical Research Unit staff at Bristol Eye Hospital. This study was funded by a block grant from Novartis UK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
CC Bailey and MA Majid have received travel funding to attend ophthalmic conferences from Novartis UK.
Rights and permissions
About this article
Cite this article
Vallance, J., Johnson, B., Majid, M. et al. A randomised prospective double-masked exploratory study comparing combination photodynamic treatment and intravitreal ranibizumab vs intravitreal ranibizumab monotherapy in the treatment of neovascular age-related macular degeneration. Eye 24, 1561–1567 (2010). https://doi.org/10.1038/eye.2010.84
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2010.84
Keywords
This article is cited by
-
Predictors of 1-year visual outcome in OCT analysis comparing ranibizumab monotherapy versus combination therapy with PDT in exsudative age-related macular degeneration
Wiener klinische Wochenschrift (2016)
-
Single-session photodynamic therapy combined with intravitreal ranibizumab for neovascular age-related macular degeneration: a comprehensive functional retinal assessment
Documenta Ophthalmologica (2013)