Abstract
Purpose
We aimed to investigate the clinical variation of rhegmatogenous retinal detachments (RD) in patients of different ethnicities.
Methods
Patients presenting with a primary RD from two ethnic groups were recruited from our tertiary referral hospital between August 2010 and December 2012. Patients who self-reported their ethnic origin either as European Caucasian (EC) or South Asian (SA) were included. Exclusion criteria included trauma, previous vitreoretinal procedures, age under 18 years, complicated cataract surgery and the presence of syndromes known to be associated with a high prevalence of RD. Detailed phenotypic data were collected. Descriptive and comparative statistical analyses were undertaken.
Results
1269 Patients were recruited. 1173 (92.4%) were EC. Mean age of onset was 58.3 years (EC) and 54.5 years (SA) (P=0.006). 75.3% EC and 58.4% SA were phakic (P<0.001). 12.8% of EC and 19.4% of SA patients had a lattice retinal degeneration in the affected eye (P=0.003). Refractive myopia was greater in SA patients (mean: −6.1DS) than EC (−4.2DS) (P=0.032). Additionally, SA patients had a greater mean axial length (25.65 mm) than EC (25.06 mm) (P=0.014). No differences were demonstrated in laterality, family history, type of retinal break or macular status.
Conclusions
SA patients present with RD at an earlier age and have a more severe phenotype than ECs. Future management strategies for RD may need to reflect these differences.
Similar content being viewed by others
Introduction
The annual risk of rhegmatogenous retinal detachments (RD) is between 6.3 and 17.9 per 100 000.1 Established risk factors include myopia,2, 3 previous cataract surgery4 and gender.5
Although the higher prevalence of certain ophthalmic conditions in alternate ethnic groups is well established,6, 7, 8, 9 the role of racial origin in RD has rarely been investigated.10 It has been suggested that Indians may have a lower incidence of RD than Caucasians.11, 12 However, the actual ocular phenotypic differences between these ethnic groups have never been described. Our work set out to investigate this between South Asians (SAs) and European Caucasians (ECs).
Materials and methods
Between August 2010 and December 2012, we prospectively collected patients of two ethnicities presenting to our unit with a primary RD. The study received local ethical review board ethical approval. Informed consent was obtained, and the study was in adherence to the tenets of the Declaration of Helsinki. As part of the Genetics in RD study, we recruited patients who self-identified as ‘EC’ or ‘SA’. The former group were defined as ethnically of European origin. The latter group was defined as ethnically originating from Pakistan, India, Sri Lanka, Bangladesh or Nepal.
An RD was defined as subretinal fluid of over two optic disc diameters, associated with at least one full thickness neurosensory retinal (NSR) break. Patients were excluded if they had undergone any intravitreal intervention (such as vitreoretinal surgery or intravitreal injections), complicated cataract surgery (associated with vitreous loss) or causative trauma. Furthermore, patients under the age of 18-years old and those with Mendelian syndromes known to have a high prevalence of RD13, 14 were excluded.
Data collected included age, gender, refractive error, laterality, axial length, lens status, presence of lattice degeneration, type of retinal break, status of macula at time of presentation and family history of RD. Descriptive and comparative statistical analyses, including χ2, Fisher’s exact and t-tests were undertaken between the two ethnic groups.
Results
1269 patients were recruited over the 28-months period of the study. This included 1173 EC and 96 SA. The cohort represents over 90% of the patients attending to our unit who fulfilled the entry criteria.
Age
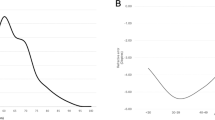
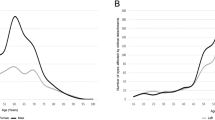
The mean age was 57.7 years (SD: 13.3 years) (Figure 1). Racial differences were evident. The EC population had a mean age of 58.3 years (SD: 13.1). The SA population had a mean age of 54.5 (SD: 13.9) and were significantly younger than the EC patients (P=0.006).
Laterality
There were an equal proportion of left and right eyes affected (631 and 624 respectively). There were 14 patients who were affected bilaterally. There was neither a significant difference regarding this laterality nor was there a difference between the ethnic groups.
Gender
There were 442 females and 827 males (1:2.84) affected. The proportion of men affected was greater in SAs (74.0%) than in Caucasians (64.5%) and suggested a significant trend (P=0.06).
Lens status
We found 75.3% of EC and 58.4% of SA were phakic with the remaining being pseudophakic. This was a statistically significant difference (P<0.001). Asian patients were more likely to be pseudophakic than Caucasians.
Break type
The break type was reported in 1187 (93.5%) of affected eyes. The most common type of NSR break was a horseshoe tear (79% in EC, 80% in SA). Other types of NSR breaks are documented in the Table 1. There was no significant difference between these groups.
Family history
A positive family history of RD (first or second degree relative) was demonstrated in 168 cases (13.2%). This was reported in 149 (12.8%) EC cases, and 19 (19.4%) SAs. There were no significant differences.
Lattice degeneration
Lattice degeneration was present in 168 patients. This was evident in 149 (12.8%) of the eyes of EC patients, and 19 (19.4%) of the eyes of SA patients (P=0.003).
Refractive error and axial length
The mean spherical equivalent for phakic affected right eyes was -4.48DS (SD 4.07). The mean axial length in all right eyes was 25.08 mm (SD 1.9). The mean spherical equivalent for phakic affected left eye was −4.18DS (SD 3.92). The mean axial length in all left eyes was 25.07 (SD 1.78). There were no significant differences between the eyes.
Comparisons between races, revealed that SA were significantly more myopic (mean −6.1DS) compared with ECs (−4.2DS) (P=0.032). Mean ocular axial lengths was 25.06 mm (standard deviation 1.82) in ECs and 25.65 mm (SD 2.30) in SA (P=0.014).
Macular status
Macular status was recorded for 1016 (86.4%) of EC and 91 (92.9%) of SA. The proportion of patients with macula on or bisecting was 50.51% (Caucasians) and 44.9% (SAs). This was not significant.
Discussion
The ocular phenotype of 1269 consecutive patients of EC or SA origin with primary RD was acquired over 28 months from the vitreoretinal department of our hospital. The majority of the patients were Caucasian (92.4%). According to the 2011 UK census,15 White British accounted for 59.8% and SA 12.1% of the population of London. Our proportion of EC patients is thus greater than suggested by the population differences.
The peak age for RD is between 60–69.16, 17, 18, 19 The incidence in this age bracket has been reported as high as 70 per 100 000 in one 20-year epidemiological study.20 Our data agrees that this age group is the peak for RD across both racial groups. The reason behind this has not been clearly explained. Although the rate of posterior vitreous detachment (PVD: the invariable pre-cursor for most horseshoe tear RD) is known to increase with age21 it has been suggested to be present in 11% of 60–69-year olds, and 46% of 80–89-year olds.22 One could infer from this that the incidence of RD should continue to increase with age. Furthermore, the pathological event of RD is likely to occur more frequently in an ‘incomplete PVD’.23 In a prospective study using modern imaging techniques to diagnose incomplete PVD, Shao et al.24 suggested that the prevalence of an incomplete PVD is higher in younger Chinese; with a minimal prevalence occurring between 75–80 years. The reason why the peak age for RD is between 60–69 years is therefore uncertain. This may suggest that PVD occurring in younger patients (60–69 years) may present with an abnormal PVD more frequently than those at a later age. Alternatively, there may simply be other health factors influencing the number of more elderly patients presenting with RD. Indeed, the life expectancy in England in 2010 was 78.2 years for men and 82.3 years for women.25 This may influence the rate.
It has been suggested that there is a bimodal incidence pattern; with a smaller peak between 20–29 years.12, 16, 26, 27 However, the largest UK epidemiological study into this condition did not replicate such a bimodal distribution.19 Our age spread (Figure 1) was in keeping with this latter epidemiological study. This suggests that the main determinant of RD is likely to be vitreous liquefaction, which increases with age.
The racial differences in age of onset are novel. This data suggests that SA patients with RD were younger than EC. Rosman and colleagues28 analysed 916 RD cases in Singapore over a four years period. The mean age for RD (for three racial groups in the study; Chinese, Malay and Indian) was 46.1 years; and this group were the first to postulate that RD may occur at a younger age in Asians (their terminology included the three races in their study). 68.1% of the 22 Indians in their cohort were aged <60-years old. A small study from India described the mean age of those with unilateral RD as 38.8 years.29
When documenting gender differences in our groups, it was clear that there were a higher number of men affected by RD (1.85:1). This trend was more marked in SA (2.84:1) compared with EC (1.81:1), which although did not reach statistical significance (P=0.06), does suggest a trend. The reason behind men being more prone to RD has often been suggested as trauma.1 However, previous studies maintain the male preponderance, when excluding for trauma.18, 19, 27 Furthermore, our cohort excluded any traumatic RD. It is possible that the higher rate of myopia seen in men, or the earlier extension of the vitreous base seen in men5, 30 may both be significant contributing factors. These differences may be more pronounced in SA.
In our cohort, 25.8% were pseudophakic. This is in agreement with the largest UK epidemiological study.19 Other large cohorts suggest this rate to range between 10–30%.26, 31 There are however reports that the proportion of pseudophakia is increasing, in line with the increase in cataract extraction over the past two decades.32, 33
It has been suggested that the association between cataract surgery and RD may be secondary to higher rates of PVD and vitreous collapse after uncomplicated cataract extraction.34, 35 What is particularly interesting is the novel finding of a higher rate of pseudophakia in SA patients with RD compared with EC (P<0.001). It is suggested that the age of onset of cataract is younger in British Asians compared with Caucasians,36 thus the rate of cataract surgery may be greater. However, as a proportion; lens extraction is a more important feature in SA than EC patients with RD in our cohort. This may be because of the vitreous differences in SA; making this group more susceptible to changes after cataract surgery. Further investigation of the vitreous anatomy in these groups could potentially elucidate differences.
Lattice degeneration was present in 13.3% of the cases. This is lower than previous historic reports.37 However, European reports range from 7–29%.38, 39 The largest UK study suggested lattice degeneration to be present in 18.7% of RD.40
The statistically significantly increased rate of retinal lattice degeneration seen in SA patients suggests a more severe phenotype. Rosman et al.28 suggest that lattice was present in 31.8% of Indians with RD (a small cohort of 22 patients). This higher prevalence of lattice degeneration may explain the younger age of SA with RD. It is likely that these data have confirmed the suggestion that lattice degeneration is more common in patients with RD from this racial group.
The myopic error found in phakic patients was not markedly different to previous reports.40 This may be secondary to the urbanised catchment area of our hospital; urbanisation has been shown to be associated with higher rates of myopia.41 Moreover, it is of interest that SA appeared to be more myopic than EC. Rosman et al.28 did not provide mean refractive error for their cohort, but 7 out of 22 (31.8%) were reported as having myopia >−5DS. We are the first to confirm that the axial length is correspondingly greater in SA patients.
Certain features of the cohort did not demonstrate significant differences between the two racial groups. In particular laterality was similar between the races, and did not confirm previous reports that RD occurs more frequently in right eyes.19, 20, 38
The proportion of patients presenting with a macula involving RD was similar to UK epidemiological studies;40 suggesting a good access to health care for all patients.
The proportion of patients who volunteered a first or second degree relative with RD is greater than previous reports (1–8% 17, 40, 42). This may reflect population differences between the various studies. Alternatively, the direct questioning in this dataset may have revealed a true higher rate. There was no difference between racial groups.
The phenotypic differences between EC and SA demonstrated here may be as a result of population stratification; a genetic phenomenon caused by non-random mating followed by a genetic drift.43 We have previously demonstrated a possible genetic association for RD in ECs.44 Investigating the genetic aetiology in different ethnicities would be of particular interest.
Our findings therefore offer novel phenotypic differences in RD between these two ethnicities. The limitations of this study must be acknowledged. All patients were recruited from one centre; thus introducing a potential bias. Furthermore self-report of ethnic origin may present limitations. However, the robust recruitment methodology gave the study significant power to help elucidate and validate the demonstrated findings.
Conclusions
Our work suggests that patients from the Indian subcontinent with RD have a more severe ocular phenotype than European Caucasians. This includes having more severe myopia, greater axial lengths, a greater proportion of retinal lattice degeneration and have a younger age of onset for the disease. Such a ‘phenotypically enriched’ cohort may prove ideal to further investigate the genetic and thus biological aetiology of RD. Furthermore, a deeper understanding of features in different populations may allow targeted health-care provision for this blinding condition.

References
Mitry D, Charteris DG, Fleck BW, Campbell H, Singh J . The epidemiology of rhegmatogenous retinal detachment: geographical variation and clinical associations. Br J Ophthalmol 2010; 94 (6): 678–684.
Burton TC . The influence of refractive error and lattice degeneration on the incidence of retinal detachment. Trans Am Ophthalmol Soc 1989; 87: 143–155.
Risk factors for idiopathic rhegmatogenous retinal detachment. The Eye Disease Case-Control Study Group. Am J Epidemiol 1993; 137 (7): 749–757.
Clark A, Morlet N, Ng JQ, Preen DB, Semmens JB . Risk for retinal detachment after phacoemulsification: a whole-population study of cataract surgery outcomes. Arch Ophthalmol 2012; 130 (7): 882–888.
Mitry D, Tuft S, McLeod D, Charteris DG . Laterality and gender imbalances in retinal detachment. Graefes Arch Clin Exp Ophthalmol 2010; 249: 1109–1110.
Qiu M, Wang SY, Singh K, Lin SC . Racial disparities in uncorrected and undercorrected refractive error in the United States. Invest Ophthalmol Vis Sci 2014; 55: 6996–7005.
Cheng JW, Zong Y, Zeng YY, Wei RL . The prevalence of primary angle closure glaucoma in adult Asians: a systematic review and meta-analysis. PLoS One 2014; 9 (7): e103222.
Scanlon PH, Aldington SJ, Stratton IM . Epidemiological issues in diabetic retinopathy. Middle East Afr J Ophthalmol 2013; 20 (4): 293–300.
Chandra A, Brookes J . Ethnicity and paediatric optic discs. Ophthalmic Physiol Opt 2012; 32 (3): 252.
Yorston D, Jalali S . Retinal detachment in developing countries. Eye 2002; 16 (4): 353–358.
Mowatt L, Shun-Shin G, Price N . Ethnic differences in the demand incidence of retinal detachments in two districts in the West Midlands. Eye 2003; 17 (1): 63–70.
Wong TY, Tielsch JM, Schein OD . Racial difference in the incidence of retinal detachment in Singapore. Arch Ophthalmol 1999; 117 (3): 379–383.
Snead MP, Clinical Yates JR . and Molecular genetics of Stickler syndrome. J Med Genet 1999; 36 (5): 353–359.
Chandra A, Ekwalla V, Child A, Charteris D . Prevalence of ectopia lentis and retinal detachment in Marfan syndrome. Acta Ophthalmol 2014; 92 (1): e82–e83.
Office National Statistics. 2015. Available at http://www.ons.gov.uk/. Accessed 08 Feb 2015.
Haimann MH, Burton TC, Brown CK . Epidemiology of retinal detachment. Arch Ophthalmol 1982; 100 (2): 289–292.
Zou H, Zhang X, Xu X, Wang X, Liu K, Ho PC . Epidemiology survey of rhegmatogenous retinal detachment in Beixinjing District, Shanghai, China. Retina 2002; 22 (3): 294–299.
Ivanisevic M, Erceg M, Eterovic D . Rhegmatogenous retinal detachment and seasonal variations. Acta Med Croatica 2002; 56 (2): 49–51.
Mitry D, Charteris DG, Yorston D, Siddiqui MA, Campbell H, Murphy AL et al. The epidemiology and socioeconomic associations of retinal detachment in Scotland: a two-year prospective population-based study. Invest Ophthalmol Vis Sci 2010; 51 (10): 4963–4968.
Rowe JA, Erie JC, Baratz KH, Hodge DO, Gray DT, Butterfield L et al. Retinal detachment in Olmsted County, Minnesota, 1976 through 1995. Ophthalmology 1999; 106 (1): 154–159.
Hayreh SS, Jonas JB . Posterior vitreous detachment: clinical correlations. Ophthalmologica 2004; 218 (5): 333–343.
Weber-Krause B, Eckardt C . [Incidence of posterior vitreous detachment in the elderly]. Ophthalmologe 1997; 94 (9): 619–623.
Sebag J . Anomalous posterior vitreous detachment: a unifying concept in vitreo-retinal disease. Graefes Arch Clin Exp Ophthalmol 2004; 242 (8): 690–698.
Shao L, Xu L, You QS, Wang YX, Chen CX, Yang H et al. Prevalence and associations of incomplete posterior vitreous detachment in adult Chinese: the Beijing Eye Study. PLoS One 2013; 8 (3): e58498.
STATISTICS OFN. 19 October 2011. Available at http://www.ons.gov.uk/ons/dcp171778_238743.pdf. Accessed 31st May 2013.
Li X . Incidence and epidemiological characteristics of rhegmatogenous retinal detachment in Beijing, China. Ophthalmology 2003; 110 (12): 2413–2417.
Polkinghorne PJ, Craig JP . Northern New Zealand Rhegmatogenous Retinal Detachment Study: epidemiology and risk factors. Clin Experiment Ophthalmol 2004; 32 (2): 159–163.
Rosman M, Wong TY, Ong SG, Ang CL . Retinal detachment in Chinese, Malay and Indian residents in Singapore: a comparative study on risk factors, clinical presentation and surgical outcomes. Int Ophthalmol 2001; 24 (2): 101–106.
Azad RV, Tewari HK, Khosla PK . Natural history of retinal detachment on the basis of the study of the fellow eye. Indian J Ophthalmol 1983; 31 (3): 170–173.
Wang J, McLeod D, Henson DB, Bishop PN . Age-dependent changes in the basal retinovitreous adhesion. Invest Ophthalmol Vis Sci 2003; 44 (5): 1793–1800.
Algvere PV, Jahnberg P, Textorius O . The Swedish Retinal Detachment Register. I. A database for epidemiological and clinical studies. Graefes Arch Clin Exp Ophthalmol 1999; 237 (2): 137–144.
Minihan M, Tanner V, Williamson TH . Primary rhegmatogenous retinal detachment: 20 years of change. Br J Ophthalmol 2001; 85 (5): 546–548.
Ducournau DH, Le Rouic JF . Is pseudophakic retinal detachment a thing of the past in the phacoemulsification era? Ophthalmology 2004; 111 (6): 1069–1070.
Ghazi NG, Green WR . Pathology and pathogenesis of retinal detachment. Eye (Lond) 2002; 16 (4): 411–421.
Boberg-Ans G, Villumsen J, Henning V . Retinal detachment after phacoemulsification cataract extraction. J Cataract Refract Surg 2003; 29 (7): 1333–1338.
Das BN, Thompson JR, Patel R, Rosenthal AR . The prevalence of age related cataract in the Asian community in Leicester: a community based study. Eye (Lond) 1990; 4 (Pt 5): 723–726.
Dumas J, Schepens CL . Chorioretinal lesions predisposing to retinal breaks. Am J Ophthalmol 1966; 61 (4): 620–630.
Laatikainen L, Tolppanen EM, Harju H . Epidemiology of rhegmatogenous retinal detachment in a Finnish population. Acta Ophthalmol (Copenh) 1985; 63 (1): 59–64.
Tornquist R, Tornquist P, Stenkula S . Retinal detachment. A study of a population-based patient material in Sweden 1971-1981. II. Pre-operative findings. Acta Ophthalmol (Copenh) 1987; 65 (2): 223–230.
Mitry D, Singh J, Yorston D, Siddiqui MA, Wright A, Fleck BW et al. The predisposing pathology and clinical characteristics in the Scottish Retinal Detachment Study. Ophthalmology 2011; 118 (7): 1429–1434.
Guo Y, Liu LJ, Xu L, Lv YY, Tang P, Feng Y et al. Outdoor activity and myopia among primary students in rural and urban regions of Beijing. Ophthalmology 2013; 120 (2): 277–283.
Wilkes SR, Beard CM, Kurland LT, Robertson DM, O'Fallon WM . The incidence of retinal detachment in Rochester, Minnesota, 1970-1978. Am J Ophthalmol 1982; 94 (5): 670–673.
Hartl DL, Clark AG . Principles of Population Genetics, 4th edn. Freeman W. H. (ed). Basingstoke: Palgrave [distributor]: New York, 2007.
Kirin M, Chandra A, Charteris DG, Hayward C, Campbell S, Celap I et al. Genome-wide association study identifies genetic risk underlying primary rhegmatogenous retinal detachment. Hum Mol Genet 2013; 22 (15): 3174–3185.
Acknowledgements
This work was supported by the Special Trustees of Moorfields Eye Hospital, The Royal College of Surgeons of Edinburgh and Fight for Sight UK and contributed towards a doctorate thesis of AC. This work was presented in part at the Royal Australian and New Zealand College of Ophthalmology (RANZCO) annual meeting (Tasmania 2013).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chandra, A., Banerjee, P., Davis, D. et al. Ethnic variation in rhegmatogenous retinal detachments. Eye 29, 803–807 (2015). https://doi.org/10.1038/eye.2015.43
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2015.43
This article is cited by
-
Epidemiology of rhegmatogenous retinal detachment in commercially insured myopes in the United States
Scientific Reports (2023)
-
Intelligent Diagnosis of Multiple Peripheral Retinal Lesions in Ultra-widefield Fundus Images Based on Deep Learning
Ophthalmology and Therapy (2023)
-
The effect of age on phenotype of primary rhegmatogenous retinal detachment
Eye (2023)
-
The effect of sex and laterality on the phenotype of primary rhegmatogenous retinal detachment
Eye (2023)
-
Retinal detachment following cataract phacoemulsification—a review of the literature
Eye (2020)