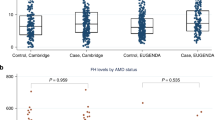
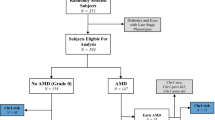
Abstract
Age-related macular degeneration (AMD) is the most common form of irreversible blindness in developed countries1,2. Variants in the factor H gene (CFH, also known as HF1), which encodes a major inhibitor of the alternative complement pathway, are associated with the risk for developing AMD3,4,5,6,7,8. Here we test the hypothesis that variation in genes encoding other regulatory proteins of the same pathway is associated with AMD. We screened factor B (BF) and complement component 2 (C2) genes, located in the major histocompatibility complex class III region, for genetic variation in two independent cohorts comprising ∼900 individuals with AMD and ∼400 matched controls. Haplotype analyses identify a statistically significant common risk haplotype (H1) and two protective haplotypes. The L9H variant of BF and the E318D variant of C2 (H10), as well as a variant in intron 10 of C2 and the R32Q variant of BF (H7), confer a significantly reduced risk of AMD (odds ratio = 0.45 and 0.36, respectively). Combined analysis of the C2 and BF haplotypes and CFH variants shows that variation in the two loci can predict the clinical outcome in 74% of the affected individuals and 56% of the controls. These data expand and refine our understanding of the genetic risk for AMD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
van Leeuwen, R., Klaver, C.C., Vingerling, J.R., Hofman, A. & de Jong, P.T. Epidemiology of age-related maculopathy: a review. Eur. J. Epidemiol. 18, 845–854 (2003).
Klein, R., Peto, T., Bird, A. & Vannewkirk, M.R. The epidemiology of age-related macular degeneration. Am. J. Ophthalmol. 137, 486–495 (2004).
Hageman, G.S. et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc. Natl. Acad. Sci. USA 102, 7227–7232 (2005).
Edwards, A.O. et al. Complement factor H polymorphism and age-related macular degeneration. Science 308, 421–424 (2005).
Haines, J.L. et al. Complement factor H variant increases the risk of age-related macular degeneration. Science 308, 419–421 (2005).
Klein, R.J. et al. Complement factor H polymorphism in age-related macular degeneration. Science 308, 385–389 (2005).
Zareparsi, S. et al. Strong association of the Y402H variant in complement factor H at 1q32 with susceptibility to age-related macular degeneration. Am. J. Hum. Genet. 77, 149–153 (2005).
Conley, Y.P. et al. Candidate gene analysis suggests a role for fatty acid biosynthesis and regulation of the complement system in the etiology of age-related maculopathy. Hum. Mol. Genet. 14, 1991–2002 (2005).
Anderson, D.H., Mullins, R.F., Hageman, G.S. & Johnson, L.V. A role for local inflammation in the formation of drusen in the aging eye. Am. J. Ophthalmol. 134, 411–431 (2002).
Hageman, G.S. et al. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch's membrane interface in aging and age-related macular degeneration. Prog. Retin. Eye Res. 20, 705–732 (2001).
Mullins, R.F., Russell, S.R., Anderson, D.H. & Hageman, G.S. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 14, 835–846 (2000).
Johnson, L.V., Leitner, W.P., Staples, M.K. & Anderson, D.H. Complement activation and inflammatory processes in Drusen formation and age related macular degeneration. Exp. Eye Res. 73, 887–896 (2001).
Crabb, J.W. et al. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc. Natl. Acad. Sci. USA 99, 14682–14687 (2002).
Bok, D. Evidence for an inflammatory process in age-related macular degeneration gains new support. Proc. Natl. Acad. Sci. USA 102, 7053–7054 (2005).
Morgan, B.P. & Walport, M.J. Complement deficiency and disease. Immunol. Today 12, 301–306 (1991).
Kinoshita, T. Biology of complement: the overture. Immunol. Today 12, 291–295 (1991).
Holers, V.M. & Thurman, J.M. The alternative pathway of complement in disease: opportunities for therapeutic targeting. Mol. Immunol. 41, 147–152 (2004).
Lokki, M.L. & Koskimies, S.A. Allelic differences in hemolytic activity and protein concentration of BF molecules are found in association with particular HLA haplotypes. Immunogenetics 34, 242–246 (1991).
Davis, C.A. & Forristal, J. Partial properdin deficiency. J. Lab. Clin. Med. 96, 633–639 (1980).
Raum, D., Glass, D., Carpenter, C.B., Schur, P.H. & Alper, C.A. Mapping of the structural gene for the second component of complement with respect to the human major histocompatibility complex. Am. J. Hum. Genet. 31, 35–41 (1979).
Alper, C.A. et al. Immunoglobulin deficiencies and susceptibility to infection among homozygotes and heterozygotes for C2 deficiency. J. Clin. Immunol. 23, 297–305 (2003).
Stewart, C.A. et al. Complete MHC haplotype sequencing for common disease gene mapping. Genome Res. 14, 1176–1187 (2004).
Larsen, C.E. & Alper, C.A. The genetics of HLA-associated disease. Curr. Opin. Immunol. 16, 660–667 (2004).
Myers, S., Bottolo, L., Freeman, C., McVean, G. & Donnelly, P.A. Fine-scale map of recombination rates and hotspots across the human genome. Science 310, 321–324 (2005).
Goverdhan, S.V. et al. Association of HLA class I and class II polymorphisms with age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 46, 1726–1734 (2005).
Bird, A.C. et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv. Ophthalmol. 39, 367–374 (1995).
Klaver, C.C. et al. Incidence and progression rates of age-related maculopathy: the Rotterdam Study. Invest. Ophthalmol. Vis. Sci. 42, 2237–2241 (2001).
Allikmets, R. et al. Mutation of the Stargardt disease gene (ABCR) in age-related macular degeneration. Science 277, 1805–1807 (1997).
Hayashi, M. et al. Evaluation of the ARMD1 locus on 1q25–31 in patients with age-related maculopathy: genetic variation in laminin genes and in exon 104 of HEMICENTIN-1. Ophthalmic Genet. 25, 111–119 (2004).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate—a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 57, 289–300 (1995).
Acknowledgements
We thank D. Anderson, L. Johnson, D. Bok, and P. Dudley for helpful discussions. We also acknowledge J. Sharp, T. Krezowik, T. Weingeist, C. Boldt, J. Folk, T. Johnson, M. Wilkinson, D. Zumbro, P. Gouras, W. Moscoso, C. McAvoy, S. Thompson, L. Arbisser and A. Arbisser for their assistance in recruiting patients; S. Baruah, R. Wolfe, S. McCormick, J. Donahue, A. Olsh, L. Buckta and M. Busuioc for technical assistance and Vision Share, the Iowa Lions Eye Bank and the Central Florida Lions Eye Banks for their efforts in procuring eyes from human donors. We are especially grateful to those individuals and families who unselfishly donated their time and/or the eyes of their loved ones to this research program. This work was supported in part by the US National Institutes of Health (NIH) (grants EY13435 (R.A.) and EY11515 (G.S.H.)), New York Community Trust (R.T.S.), Wallach Foundation (R.A., G.R.B.), Elyachar Foundation (R.A., G.R.B.), Kaplen Foundation (R.A., G.R.B.), Widgeon Point Charitable Foundation (R.A., J.E.M.), Macula Foundation (R.A.), the International Retina Research Foundation (G.S.H.), the American Macular Degeneration Foundation, Inc. (G.S.H.), the Eye Research Institute (G.S.H.), the Intramural Research Program of the NIH and the National Cancer Institute; by federal funds from the National Cancer Institute of the National Institutes of Health (contract NO1-CO-124000) and by unrestricted grants from Research to Prevent Blindness, Inc., to the University of Iowa Department of Ophthalmology and Visual Sciences and to the Department of Ophthalmology, Columbia University. G.S.H. currently holds an honorary professorship in the School of Medicine, Queen's University, Belfast. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the US Government.
Author information
Authors and Affiliations
Consortia
Corresponding authors
Ethics declarations
Competing interests
K.C. and J.N. are employees of Sapio Sciences LLC, York, Pennsylvania, USA.
Additional information
The AMD Genetics Clinical Study Group includes
Supplementary information
Supplementary Fig. 1
Linkage disequilibrium map of the complement region. (PDF 298 kb)
Rights and permissions
About this article
Cite this article
Gold, B., Merriam, J., Zernant, J. et al. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet 38, 458–462 (2006). https://doi.org/10.1038/ng1750
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1750
This article is cited by
-
C1q and the classical complement cascade in geographic atrophy secondary to age-related macular degeneration
International Journal of Retina and Vitreous (2022)
-
The effect of systemic levels of TNF-alpha and complement pathway activity on outcomes of VEGF inhibition in neovascular AMD
Eye (2022)
-
Complement Mediators in Development to Treat Age-Related Macular Degeneration
Drugs & Aging (2022)
-
Therapeutic Options Under Development for Nonneovascular Age-Related Macular Degeneration and Geographic Atrophy
Drugs & Aging (2021)
-
Association of imaging biomarkers and local activation of complement in aqueous humor of patients with early forms of age-related macular degeneration
Graefe's Archive for Clinical and Experimental Ophthalmology (2021)