Abstract
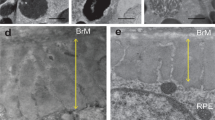
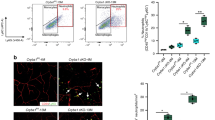
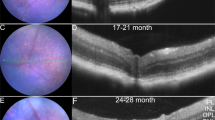
The study and treatment of age-related macular degeneration (AMD), a leading cause of blindness, has been hampered by a lack of animal models. Here we report that mice deficient either in monocyte chemoattractant protein-1 (Ccl-2; also known as MCP-1) or its cognate C-C chemokine receptor-2 (Ccr-2) develop cardinal features of AMD, including accumulation of lipofuscin in and drusen beneath the retinal pigmented epithelium (RPE), photoreceptor atrophy and choroidal neovascularization (CNV). Complement and IgG deposition in RPE and choroid accompanies senescence in this model, as in human AMD. RPE or choroidal endothelial production of Ccl-2 induced by complement C5a and IgG may mediate choroidal macrophage infiltration into aged wild-type choroids. Wild-type choroidal macrophages degrade C5 and IgG in eye sections of Ccl2−/− or Ccr2−/− mice. Impaired macrophage recruitment may allow accumulation of C5a and IgG, which induces vascular endothelial growth factor (VEGF) production by RPE, possibly mediating development of CNV. These models implicate macrophage dysfunction in AMD pathogenesis and may be useful as a platform for validating therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Smith, W. et al. Risk factors for age-related macular degeneration: pooled findings from three continents. Ophthalmology 108, 697–704 (2001).
Dithmar, S. et al. Murine high-fat diet and laser photochemical model of basal deposits in Bruch membrane. Arch. Ophthalmol. 119, 1643–1649 (2001).
Cousins, S.W. et al. The role of aging, high fat diet and blue light exposure in an experimental mouse model for basal laminar deposit formation. Exp. Eye Res. 75, 543–553 (2002).
Majji, A.B. et al. Age-related retinal pigment epithelium and Bruch's membrane degeneration in senescence-accelerated mouse. Invest. Ophthalmol. Vis. Sci. 41, 3936–3942 (2000).
Weng, J. et al. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt's disease from the phenotype in abcr knockout mice. Cell 98, 13–23 (1999).
Dithmar, S., Curcio, C.A., Le, N.A., Brown, S. & Grossniklaus, H.E. Ultrastructural changes in Bruch's membrane of apolipoprotein E-deficient mice. Invest. Ophthalmol. Vis. Sci. 41, 2035–2042 (2000).
Rakoczy, P.E. et al. Progressive age-related changes similar to age-related macular degeneration in a transgenic mouse model. Am. J. Pathol. 161, 1515–1524 (2002).
Lu, B. et al. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J. Exp. Med. 187, 601–608 (1998).
Kuziel, W.A. et al. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc. Natl. Acad. Sci. USA 94, 12053–12058 (1997).
Green, W.R. & Enger, C. Age-related macular degeneration histopathologic studies. The 1992 Lorenz E. Zimmerman Lecture. Ophthalmology 100, 1519–1535 (1993).
Kamei, M. & Hollyfield, J.G. TIMP-3 in Bruch's membrane: changes during aging and in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 40, 2367–2375 (1999).
Delori, F.C., Fleckner, M.R., Goger, D.G., Weiter, J.J. & Dorey, C.K. Autofluorescence distribution associated with drusen in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 41, 496–504 (2000).
Eldred, G.E. & Lasky, M.R. Retinal age pigments generated by self-assembling lysosomotropic detergents. Nature 361, 724–726 (1993).
Suter, M. et al. Age-related macular degeneration. The lipofuscin component N-retinyl-N-retinylidene ethanolamine detaches proapoptotic proteins from mitochondria and induces apoptosis in mammalian retinal pigment epithelial cells. J. Biol. Chem. 275, 39625–39630 (2000).
Finnemann, S.C., Leung, L.W. & Rodriguez-Boulan, E. The lipofuscin component A2E selectively inhibits phagolysosomal degradation of photoreceptor phospholipid by the retinal pigment epithelium. Proc. Natl. Acad. Sci. USA 99, 3842–3847 (2002).
Ryan, S.J. Subretinal neovascularization. Natural history of an experimental model. Arch. Ophthalmol. 100, 1804–1809 (1982).
Tobe, T. et al. Targeted disruption of the FGF2 gene does not prevent choroidal neovascularization in a murine model. Am. J. Pathol. 153, 1641–1646 (1998).
Spilsbury, K., Garrett, K.L., Shen, W.Y., Constable, I.J. & Rakoczy, P.E. Overexpression of vascular endothelial growth factor (VEGF) in the retinal pigment epithelium leads to the development of choroidal neovascularization. Am. J. Pathol. 157, 135–144 (2000).
Baffi, J., Byrnes, G., Chan, C.C. & Csaky, K.G. Choroidal neovascularization in the rat induced by adenovirus mediated expression of vascular endothelial growth factor. Invest. Ophthalmol. Vis. Sci. 41, 3582–3589 (2000).
Schwesinger, C. et al. Intrachoroidal neovascularization in transgenic mice overexpressing vascular endothelial growth factor in the retinal pigment epithelium. Am. J. Pathol. 158, 1161–1172 (2001).
Mullins, R.F., Russell, S.R., Anderson, D.H. & Hageman, G.S. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 14, 835–846 (2000).
Johnson, L.V., Ozaki, S., Staples, M.K., Erickson, P.A. & Anderson, D.H. A potential role for immune complex pathogenesis in drusen formation. Exp. Eye Res. 70, 441–449 (2000).
Anderson, D.H., Mullins, R.F., Hageman, G.S. & Johnson, L.V. A role for local inflammation in the formation of drusen in the aging eye. Am. J. Ophthalmol. 134, 411–431 (2002).
Ishibashi, T. et al. Advanced glycation end products in age-related macular degeneration. Arch. Ophthalmol. 116, 1629–1632 (1998).
Schulze, M. et al. Glomerular C3c localization indicates ongoing immune deposit formation and complement activation in experimental glomerulonephritis. Am. J. Pathol. 142, 179–187 (1993).
Brons, R.H. et al. Detection of immune deposits in skin lesions of patients with Wegener's granulomatosis. Ann. Rheum. Dis. 60, 1097–1102 (2001).
Bian, Z.M. et al. Glycated serum albumin induces chemokine gene expression in human retinal pigment epithelial cells. J. Leukoc. Biol. 60, 405–414 (1996).
Duvall, J. & Tso, M.O. Cellular mechanisms of resolution of drusen after laser coagulation. An experimental study. Arch. Ophthalmol. 103, 694–703 (1985).
Riedemann, N.C. et al. Expression and function of the C5a receptor in rat alveolar epithelial cells. J. Immunol. 168, 1919–1925 (2002).
Lu, M. et al. Advanced glycation end products increase retinal vascular endothelial growth factor expression. J. Clin. Invest. 101, 1219–1224 (1998).
Hoffmann, S., Friedrichs, U., Eichler, W., Rosenthal, A. & Wiedemann, P. Advanced glycation end products induce choroidal endothelial cell proliferation, matrix metalloproteinase-2 and VEGF upregulation in vitro. Graefes Arch. Clin. Exp. Ophthalmol. 240, 996–1002 (2002).
Roberts, W.G. & Palade, G.E. Neovasculature induced by vascular endothelial growth factor is fenestrated. Cancer Res. 57, 765–772 (1997).
Penfold, P.L., Provis, J.M., Furby, J.H., Gatenby, P.A. & Billson, F.A. Autoantibodies to retinal astrocytes associated with age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 228, 270–274 (1990).
Gurne, D.H., Tso, M.O., Edward, D.P. & Ripps, H. Antiretinal antibodies in serum of patients with age-related macular degeneration. Ophthalmology 98, 602–607 (1991).
Mullins, R.F., Aptsiauri, N. & Hageman, G.S. Structure and composition of drusen associated with glomerulonephritis: implications for the role of complement activation in drusen biogenesis. Eye 15, 390–395 (2001).
Leys, A. et al. Subretinal neovascular membranes associated with chronic membranoproliferative glomerulonephritis type II. Graefes Arch. Clin. Exp. Ophthalmol. 228, 499–504 (1990).
Ishida, O. et al. Is Chlamydia pneumoniae infection a risk factor for age related macular degeneration? Br. J. Ophthalmol. 87, 523–524 (2003).
Kalayoglu, M.V., Galvan, C., Mahdi, O.S., Byrne, G.I. & Mansour, S. Serological association between Chlamydia pneumoniae infection and age-related macular degeneration. Arch. Ophthalmol. 121, 478–482 (2003).
Kothe, H. et al. Hydroxymethylglutaryl coenzyme A reductase inhibitors modify the inflammatory response of human macrophages and endothelial cells infected with Chlamydia pneumoniae. Circulation 101, 1760–1763 (2000).
Meda, L. et al. β-amyloid (25-35) peptide and interferon-γ synergistically induce the production of the chemotactic cytokine MCP-1/JE in monocytes and microglial cells. J. Immunol. 157, 1213–1218 (1996).
Boring, L., Gosling, J., Cleary, M. & Charo, I.F. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 394, 894–897 (1998).
Gu, L. et al. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol. Cell 2, 275–281 (1998).
Penfold, P.L., Madigan, M.C., Gillies, M.C. & Provis, J.M. Immunological and aetiological aspects of macular degeneration. Prog. Retin. Eye Res. 20, 385–414 (2001).
Grossniklaus, H.E. et al. Macrophage and retinal pigment epithelium expression of angiogenic cytokines in choroidal neovascularization. Mol. Vis. 8, 119–126 (2002).
Sakurai, E. et al. Targeted disruption of the CD18 or ICAM-1 gene inhibits choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 44, 2743–2749 (2003).
Sakurai, E., Anand, A., Ambati, B.K., van Rooijen, N. & Ambati, J. Macrophage depletion inhibits experimental choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 44, 3578–3585 (2003).
Ambati, J., Ambati, B.K., Yoo, S.H., Ianchulev, S. & Adamis, A.P. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv. Ophthalmol. 48, 257–293 (2003).
Acknowledgements
We thank M.G. Engle, M. Jennes and R. King for assistance with histology and electron microscopy; G. Chen for maintaining cell cultures; M.H. Hanson for photography; J. Husemann for technical advice; H.E. Grossniklaus for expertise in interpreting electron micrographs; and A.P. Adamis, S. Bondada, D.C. Collins, R. Mohan, E.J. Smart, V. Kumar, P.A. Pearson, A.M. Rao, G.S. Rao and J.G. Woodward for valuable discussions. J.A. was supported by a Foundation Fighting Blindness Career Development Award, the Dennis W. Jahnigen Career Development Award administered by the American Geriatrics Society and funded by the John A. Hartford Foundation and Atlantic Philanthropies, grants from Prevent Blindness America and Fight for Sight and a physician-scientist award from University of Kentucky; E.S. was supported by a Fight For Sight postdoctoral fellowship; and B.K.A. was supported by a Knights-Templar Eye Foundation grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
J.A. is listed on an initial patent filing by the University of Kentucky, describing the findings in these mice.
Rights and permissions
About this article
Cite this article
Ambati, J., Anand, A., Fernandez, S. et al. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat Med 9, 1390–1397 (2003). https://doi.org/10.1038/nm950
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm950
This article is cited by
-
Oxidative stress and epigenetics in ocular vascular aging: an updated review
Molecular Medicine (2023)
-
Forward genetic screening using fundus spot scale identifies an essential role for Lipe in murine retinal homeostasis
Communications Biology (2023)
-
Homeostasis and dyshomeostasis of the retina
Current Medicine (2023)
-
SARS-COV-2 spike protein promotes RPE cell senescence via the ROS/P53/P21 pathway
Biogerontology (2023)
-
Update of application of olfactory ensheathing cells and stem cells/exosomes in the treatment of retinal disorders
Stem Cell Research & Therapy (2022)