Key Points
-
The oculomotor system is a useful model for the study of purposeful movements. Rotations of the eyes are produced by three pairs of extraocular muscles that are innervated by motor neurons from the III, IV and VI cranial nerve nuclei. A saccadic eye movement is produced when the appropriate motor neurons produce a burst of spikes, followed by tonic firing at the correct rate to maintain the eye's new position.
-
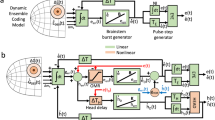
The commands for horizontal components of saccades originate in the pons and medulla. Omnipause neurons fire tonically during fixation, but stop firing during saccades; long-lead burst neurons and excitatory burst neurons fire before and during saccades. Excitatory burst neurons synapse onto motor neurons and drive the motor neuron pulse of activity. Neurons in the prepositus and vestibular nuclei fire tonically and drive the step of activity that maintains eye position. Microstimulation of neurons in the pontine reticular formation produces horizontal eye movements. Neurons in the rostral midbrain that have similar properties drive vertical eye movements. The duration and velocity of saccades are determined by the duration and maximal firing rate of the bursts of activity produced by these neurons.
-
The horizontal and vertical components of oblique saccades are coordinated. The shorter of the two components proceeds at a lower velocity than normal so that the two will have the same duration and the movement will not be curved. The onsets of the two components are coordinated by omnipause neurons in the pons.
-
The torsional component of saccadic eye movements is stereotyped and obeys Donders' law: for any direction of the line of sight, if the head is upright and stationary, the eye will assume a given degree of torsion, regardless of the route taken by the eye to reach its position. Saccades also obey Listing's law, which specifies the orientation of the globe for each gaze position. It is unclear how these laws are implemented, but identifying the mechanisms is an important part of understanding the generation of saccades.
-
Neurons in the superior colliculus (SC) provide the main input to the pontine and midbrain pulse–step generators. Microstimulation of these neurons produces saccades in head-restrained animals, and coordinated head and eye movements in head-unrestrained animals. The size and direction of the movements produced are primarily determined by the position of the stimulation in the SC.
-
Models of saccade generation assume that saccades are under feedback control and that the feedback comes from corollary discharge. Models differ in the implementation of this feedback, but it has not been possible to determine the type of feedback signal used or the site of the comparator. Reasons for this include the fact that signals that are separate in models might be intermingled in the brain, preventing selective lesioning of these signals, and that different models produce similar signals. Models also tend to use population signals, but in terms of electrophysiology, it is hard to know what the population signal is at any given time. The problems faced by oculomotor researchers are mirrored in other areas of systems neuroscience.
Abstract
The modern era of oculomotor research began with the advent of the chronic single-unit recording method in the late 1960s. Research carried out in the intervening years has made it possible to provide a detailed description of the saccadic command signals that are generated by motor neurons and the formation of these signals in premotor brainstem regions. These findings have been assimilated in control-systems models that simulate important behavioural features of saccades. Despite these great advances, key issues, such as the nature of the feedback signal and the location of the comparator, are unresolved and some of the factors that have impeded progress can be identified.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Fuchs, A. F. & Luschei, E. S. Firing patterns of abducens neurons of alert monkeys in relationship to horizontal eye movement. J. Neurophysiol. 33, 382–392 (1970).
Robinson, D. A. Oculomotor unit behavior in the monkey. J. Neurophysiol. 33, 393–404 (1970).
Schiller, P. H. The discharge characteristics of single units in the oculomotor and abducens nuclei of the unanesthetized monkey. Exp. Brain Res. 10, 347–362 (1970).
Fuchs, A. F. & Luschei, E. S. The activity of single trochlear nerve fibers during eye movements in the alert monkey. Exp. Brain Res. 13, 78–89 (1971). References 1–4 were the first to describe the behaviour of extraocular muscle motor neurons in awake, behaving animals. The findings were in remarkable agreement on the general properties of motor neurons.
Sylvestre, P. A. & Cullen, K. E. Quantitative analysis of abducens neuron discharge dynamics during saccadic and slow eye movements. J. Neurophysiol. 82, 2612–2632 (1999). This paper contains a contemporary description of the quantitative properties of motor neuron activity.
Sparks, D. L. & Travis, R. P. Firing patterns of reticular neurons during horizontal eye movements. Brain Res. 33, 477–481 (1971).
Cohen, B. & Henn, V. Unit activity in the pontine reticular formation associated with eye movements. Brain Res. 46, 403–410 (1972).
Luschei, E. S. & Fuchs, A. F. Activity of brain stem neurons during eye movements of alert monkeys. J. Neurophysiol. 35, 445–461 (1972).
Keller, E. L. Participation of medial pontine reticular formation in eye movement generation in monkey. J. Neurophysiol. 37, 316–332 (1974).
Henn, V. & Cohen, B. Coding of information about rapid eye movements in the pontine reticular formation of alert monkeys. Brain Res. 108, 307–325 (1976).
Becker, W. in Eye Movements (ed. Carpenter, R. H. S.) 95–137 (CRC Press, Boca Raton, Florida, 1991). A summary of psychophysical data on the properties of saccades.
Robinson, D. A. The mechanics of human saccadic eye movement. J. Physiol. (Lond.) 174, 245–264 (1964).
Hepp, K., Henn, V., Vilis, T. & Cohen, B. in The Neurobiology of Saccadic Eye Movements (eds Wurtz, R. H. & Goldberg, M. E.) 105–212 (Elsevier, Amsterdam, 1989). An excellent review that provides important background information about reference frames and other fundamental topics.
Moschovakis, A. K., Scudder, C. A. & Highstein, S. M. The microscopic anatomy and physiology of the mammalian saccadic system. Prog. Neurobiol. 50, 133–254 (1996). The unabridged source of detailed information about the structural and functional properties of neurons involved in the control of saccades.
Scudder, C. A., Kaneko, C. R. S. & Fuchs, A. F. The brainstem burst generator for saccadic eye movements: a modern synthesis. Exp. Brain Res. 142, 439–462 (2002). A more recent review of the brainstem mechanisms involved in saccade generation.
Buttner, U., Buttner-Ennever, J. A. & Henn, V. Vertical eye movement related unit activity in the rostral mesencephalic reticular formation of the alert monkey. Brain Res. 130, 239–252 (1977).
King, W. M. & Fuchs, A. F. in Control of Gaze by Brain Stem Neurons (eds Baker, R. & Berthoz, A.) 319–326 (Elsevier, Amsterdam, 1977).
Buttner-Ennever, J. A. & Buttner, U. A cell group associated with vertical eye movements in the rostral mesencephalic reticular formation of the monkey. Brain Res. 151, 31–47 (1978).
King, W. M. & Fuchs, A. F. Reticular control of vertical saccadic eye movements by mesencephalic burst neurons. J. Neurophysiol. 42, 861–876 (1979).
King, W. M., Fuchs, A. F. & Magnin, M. Vertical eye movement-related responses of neurons in midbrain near interstitial nucleus of Cajal. J. Neurophysiol. 46, 549–562 (1981).
Kokkoroyannis, R., Scudder, C. A., Highstein, S. M., Balaban, C. & Moschovakis, A. K. The anatomy and physiology of the primate interstitial nucleus of Cajal. I. Efferent projections. J. Neurophysiol. 75, 725–739 (1996).
Dalezios, Y., Scudder, C. A., Highstein, S. M. & Moschovakis, A. K. Anatomy and physiology of the primate interstitial nucleus of Cajal. II. Discharge pattern of single efferent fibers. J. Neurophysiol. 80, 3100–3111 (1998).
Igusa, Y., Sasaki, S. & Shimazu, H. Excitatory premotor burst neurons in the cat pontine reticular formation related to the quick phase of vestibular nystagmus. Brain Res. 182, 451–456 (1980).
Strassman, A., Highstein, S. M. & McCrea, R. A. Anatomy and physiology of saccadic burst neurons in the alert squirrel monkey. I. Excitatory burst neurons. J. Comp. Neurol. 249, 337–357 (1986).
Cohen, B. & Komatsuzaki, A. Eye movements induced by stimulation of the pontine reticular formation: evidence for integration in oculomotor pathways. Exp. Neurol. 36, 101–117 (1972).
Sparks, D. L., Barton, E. J., Gandhi, N. J. & Nelson, J. Studies of the role of the paramedian pontine reticular formation (PPRF) in the control of head-restrained and head-unrestrained gaze shifts. Ann. NY Acad. Sci. 956, 85–98 (2002).
Curthoys, I. S., Markham, C. H. & Furuya, N. Direct projection of pause neurons to nystagmus-related excitatory burst neurons in the cat pontine reticular formation. Exp. Neurol. 83, 414–422 (1984).
Guitton, D. & Mandl, G. Oblique saccades of the cat: a comparison between the durations of horizontal and vertical components. Vision Res. 20, 875–881 (1980).
King, W. M., Lisberger, S. G. & Fuchs, A. F. Oblique saccadic eye movements of primates. J. Neurophysiol. 56, 769–784 (1986).
Becker, W. & Jurgens, R. Human oblique saccades: quantitative analysis of the relation between horizontal and vertical components. Vision Res. 30, 893–920 (1990).
Smit, A. C., Van Opstal, A. J. & Van Gisbergen, J. A. Component stretching in fast and slow oblique saccades in the human. Exp. Brain Res. 81, 325–334 (1990).
Suzuki, Y. et al. Three-dimensional extraocular motoneuron innervation in the rhesus monkey. I. Muscle rotation axes and on-directions during fixation. Exp. Brain Res. 126, 187–199 (1999).
Vilis, T., Hepp, K., Schwarz, U. & Henn, V. On the generation of vertical and torsional rapid eye movements in the monkey. Exp. Brain Res. 77, 1–11 (1989).
Crawford, J. D. & Vilis, T. Symmetry of oculomotor burst neuron coordinates about Listing's plane. J. Neurophysiol. 68, 432–448 (1992). The most comprehensive description of the effects of microstimulation and pharmacological inactivation of neurons in the riMLF. An important discussion of the axes of eye rotation generated by populations of burst neurons and the coordinate system defined by these neurons.
Helmchen, C., Rambold, H. & Buttner, U. Saccade-related burst neurons with torsional and vertical on-directions in the interstitial nucleus of Cajal of the alert monkey. Exp. Brain Res. 112, 63–78 (1996).
Helmchen, C., Rambold, H., Fuhry, L. & Buttner, U. Deficits in vertical and torsional eye movements after uni- and bilateral muscimol inactivation of the interstitial nucleus of Cajal of the alert monkey. Exp. Brain Res. 119, 436–452 (1998).
Sparks, D. L. & Hartwich-Young, R. in The Neurobiology of Saccadic Eye Movements (eds Wurtz, R. H. & Goldberg, M. E.) 213–255 (Elsevier, Amsterdam, 1989).
Sparks, D. L. The neural translation of sensory signals into commands for the control of saccadic eye movements: the role of the primate superior colliculus. Physiol. Rev. 66, 118–171 (1986). References 37 and 38 summarize the results of experiments testing the effects of combined cortical and collicular lesions.
Wurtz, R. H. & Goldberg, M. E. Activity of superior colliculus in behaving monkey. IV. Effects of lesions on eye movement. J. Neurophysiol. 35, 587–596 (1972).
Schiller, P. H., True, S. D. & Conway, J. L. Deficits in eye movements following frontal eye-field and superior colliculus ablations. J. Neurophysiol. 44, 1175–1189 (1980).
Hikosaka, O. & Wurtz, R. H. Modification of saccadic eye movements by GABA-related substances. I. Effect of muscimol and bicuculline in monkey superior colliculus. J. Neurophysiol. 53, 266–291 (1985).
Lee, C., Rohrer, W. H. & Sparks, D. L. Population coding of saccadic eye movements by neurons in the superior colliculus. Nature 332, 357–360 (1988).
Aizawa, H. & Wurtz, R. H. Reversible inactivation of monkey superior colliculus. I. Curvature of saccadic trajectory. J. Neurophysiol. 79, 2082–2096 (1998).
Quaia, C., Aizawa, H., Optican, L. M. & Wurtz, R. H. Reversible inactivation of monkey superior colliculus. II. Maps of saccadic deficits. J. Neurophysiol. 79, 2097–2110 (1998).
Hanes, D. P. & Wurtz, R. H. Interaction of the frontal eye field and superior colliculus for saccade generation. J. Neurophysiol. 85, 804–815 (2001).
Robinson, D. A. Eye movements evoked by collicular stimulation in the alert monkey. Vision Res. 12, 1795–1808 (1972).
Schiller, P. H. & Stryker, M. Single-unit recording and stimulation in superior colliculus of the alert rhesus monkey. J. Neurophysiol. 35, 915–924 (1972).
Pare, M., Crommelinck, M. & Guitton, D. Gaze shifts evoked by stimulation of the superior colliculus in the head-free cat conform to the motor map but also depend on stimulus strength and fixation activity. Exp. Brain Res. 101, 123–139 (1994).
Stanford, T. R., Freedman, E. G. & Sparks, D. L. The site and parameters of microstimulation determine the properties of eye movements evoked from the primate superior colliculus: evidence for independent collicular signals of saccade displacement and velocity. J. Neurophysiol. 76, 3360–3381 (1996).
Wurtz, R. H. & Goldberg, M. E. Activity of superior colliculus in behaving monkey. III. Cells discharging before eye movements. J. Neurophysiol. 35, 575–596 (1972).
Sparks, D. L., Holland, R. & Guthrie, B. L. Size and distribution of movement fields in the monkey superior colliculus. Brain Res. 113, 21–34 (1976).
Sparks, D. L., Lee, C. & Rohrer, W. H. Population coding of the direction, amplitude, and velocity of saccadic eye movements by neurons in the superior colliculus. Cold Spring Harb. Symp. Quant. Biol. 55, 805–811 (1990).
Sparks, D. L., Kristan, W. B. & Shaw, B. K. in Neurons, Networks, and Motor Behavior (eds Stein, P. S. G., Grillner, S., Selverston, A. I. & Stuart, D. G.) 21–32 (MIT Press, Cambridge, Massachusetts, 1997).
Freedman, E. G., Stanford, T. R. & Sparks, D. L. Combined eye-head gaze shifts produced by electrical stimulation of the superior colliculus in rhesus monkeys. J. Neurophysiol. 76, 927–952 (1996).
Klier, E. M., Wang, H. & Crawford, J. D. Neural mechanisms of three-dimensional eye and head movements. Ann. NY Acad. Sci. 956, 512–514 (2002).
van Opstal, A. J., Hepp, K., Hess, B. J., Straumann, D. & Henn, V. Two- rather than three-dimensional representation of saccades in monkey superior colliculus. Science 252, 1313–1315 (1991).
Hepp, K., Van Opstal, A. J., Straumann, D., Hess, B. J. & Henn, V. Monkey superior colliculus represents rapid eye movements in a two-dimensional motor map. J. Neurophysiol. 69, 965–979 (1993).
Sparks, D. L. Conceptual issues related to the role of the superior colliculus in the control of gaze. Curr. Opin. Neurobiol. 9, 698–707 (1999).
Munoz, D. P., Guitton, D. & P´lisson, D. Control of orienting gaze shifts by tectoreticulospinal system in the head-free cat. III. Spatiotemporal characteristics of phasic motor discharges. J. Neurophysiol. 66, 642–666 (1991).
Freedman, E. G. & Sparks, D. L. Activity of cells in the deeper layers of the superior colliculus of rhesus monkey: evidence for a gaze displacement command. J. Neurophysiol. 78, 1669–1690 (1997).
Sparks, D. L., Rohrer, B. & Zhang, Y. The role of the superior colliculus in saccade initiation: a study of express saccades and the gap effect. Vision Res. 40, 2763–2777 (2000).
Moschovakis, A. K. et al. An anatomical substrate for the spatiotemporal transformation. J. Neurosci. 18, 10219–10229 (1998). The most comprehensive attempt to test the hypothesis that the metrics of saccades caused by the activation of neurons in particular regions of the collicular map depend on the strength of their projections onto the horizontal and vertical burst generators.
Zee, D. S., Optican, L. M., Cook, J. D., Robinson, D. A. & Engel, W. K. Slow saccades in spinocerebellar degeneration. Arch. Neurol. 33, 243–251 (1976).
Robinson, D. A. in Basic Mechanisms of Ocular Motility and their Clinical Implications (eds Lennerstrand, G. & Bach-y-Rita, P.) 337–374 (Pergamon, Oxford, UK, 1975).
Keller, E. L. in Control of Gaze by Brain Stem Neurons (eds Baker, R. & Berthoz, A.) 327–336 (Elsevier, Amsterdam, 1977).
Keller, E. L. & Edelman, J. A. Use of interrupted saccade paradigm to study spatial and temporal dynamics of saccadic burst cells in superior colliculus in monkey. J. Neurophysiol. 72, 2754–2770 (1994).
Keller, E. L., Gandhi, N. J. & Shieh, J. M. Endpoint accuracy in saccades interrupted by stimulation in the omnipause region in monkey. Vis. Neurosci. 13, 1059–1067 (1996).
Guthrie, B. L., Porter, J. D. & Sparks, D. L. Corollary discharge provides accurate eye position information to the oculomotor system. Science 221, 1193–1195 (1983).
Fukushima, K., Kaneko, C. R. S. & Fuchs, A. F. The neuronal substrate of integration in the oculomotor system. Prog. Neurobiol. 39, 609–639 (1992).
Moschovakis, A. K. The neural integrators of the mammalian saccadic system. Front. Biosci. 2, 552–577 (1997). References 69 and 70 summarize what is known about the neural underpinnings of integration in the oculomotor system.
Tweed, D. & Vilis, T. A two dimensional model for saccade generation. Biol. Cybern. 52, 219–227 (1985).
van Gisbergen, J. A. M., van Opstal, A. J. & Schoenmakers, J. J. M. Experimental test of two models for the generation of oblique saccades. Exp. Brain Res. 57, 321–336 (1985).
Grossman, G. E. & Robinson, D. A. Ambivalence in modelling oblique saccades. Biol. Cybern. 58, 13–18 (1988).
Becker, W. & Jurgens, R. Human oblique saccades: quantitative analysis of the relation between horizontal and vertical components. Vision Res. 30, 893–920 (1990).
Tweed, D. & Vilis, T. Implications of rotational kinematics for the oculomotor system in three dimensions. J. Neurophysiol. 58, 832–849 (1987).
Tweed, D. & Vilis, T. The superior colliculus and spatiotemporal translation in the saccadic system. Neural Netw. 3, 75–86 (1990).
Demer, J. L. The orbital pulley system: a revolution in concepts of orbital anatomy. Ann. NY Acad. Sci. 956, 17–32 (2002). A summary of data related to the orbital pulley system with a discussion of the implications for studies of the neural control of eye movements.
Raphan, T. Modeling control of eye orientation in three dimensions. I. Role of muscle pulleys in determining saccadic trajectory. J. Neurophysiol. 79, 2653–2667 (1998).
Porrill, J., Warren, P. A. & Dean P. A simple control law generates Listing's positions in a detailed model of the extraocular muscle system. Vision Res. 40, 3743–3758 (2000).
Tweed, D., Haslwanter, T. & Fetter, M. Optimizing gaze control in three dimensions. Science 281, 1363–1366 (1998).
Tweed, D. B., Haslwanter, T. P., Happe, V. & Fetter, M. Non-commutativity in the brain. Nature 399, 261–263 (1999).
Misslisch, H. & Hess, B. J. M. Three-dimensional vestibuloocular reflex of the monkey: optimal retinal image stabilization versus listing's law. J. Neurophysiol. 83, 3264–3276 (2000).
Haslwanter, T. Mechanics of eye movements: implications of the 'orbital revolution'. Ann. NY Acad. Sci. 956, 17–32 (2002). A useful overview of recent models that were developed to understand the implications of muscle pulleys for the neural control of eye movements.
Galiana, H. L. & Guitton, D. Central organization and modeling of eye–head coordination during orienting gaze shifts. Ann. NY Acad. Sci. 22, 452–471 (1992).
Guitton, D., Munoz, D. P. & Galiana, H. L. Gaze control in the cat: studies and modeling of the coupling between orienting eye and head movements in different behavioral tasks. J. Neurophysiol. 64, 509–531 (1990).
Cheron, G., Godaux, E., Laune, J. M. & Vanderkelen, B. Lesions in the cat prepositus complex: effects on the vestibulo-ocular reflex and saccades. J. Physiol. (Lond.) 372, 75–94 (1986).
Cannon, S. C. & Robinson, D. A. Loss of the neural integrator of the oculomotor system from brain stem lesions in monkey. J. Neurophysiol. 57, 1383–1409 (1987).
Cheron, G. & Godaux, E. Disabling of the oculomotor neural integrator by kainic acid injections in the prepositus-vestibular complex of the cat. J. Physiol. (Lond.) 394, 267–290 (1987).
Kaneko, C. R. S. Eye movement deficits following ibotenic acid lesions of the nucleus prepositus hypoglossi in monkeys. I. Saccades and fixation. J. Neurophysiol. 78, 1753–1768 (1997).
Scudder, C. A. A new local feedback model of the saccadic burst generator. J. Neurophysiol. 59, 1455–1475 (1988).
Bozis, A. & Moschovakis, A. K. Neural network simulations of the primate oculomotor system. III. An one-dimensional, one-directional model of the superior colliculus. Biol. Cybern. 79, 215–230 (1998).
Van Gisbergen, J. A. M., Robinson, D. A. & Gielen, S. A quantitative analysis of generation of saccadic eye movements by burst neurons. J. Neurophysiol. 45, 417–442 (1981).
Yoshida, K., Iwamoto, Y., Chimoto, S. & Shimazu, H. Saccade-related inhibitory input to pontine omnipause neurons: an intracellular study in alert cats. J. Neurophysiol. 82, 1198–1208 (1999).
Collins, C. C. in Basic Mechanisms of Ocular Motility and their Clinical Implications (eds Lennerstrand, G. & Bach-y-Rita, P.) 145–180 (Pergamon, Oxford, UK, 1975).
Robinson, D. A. A quantitative analysis of extraocular muscle cooperation and squint. Invest. Ophthalmol. 14, 801–825 (1975).
Optican, L. M. & Robinson, D. A. Cerebellar-dependent adaptive control of primate saccadic system. J. Neurophysiol. 44, 1058–1076 (1980).
Dean, P. Motor unit recruitment in a distributed model of extraocular muscle. J. Neurophysiol. 76, 727–742 (1996).
Dean, P. Simulated recruitment of medial rectus motoneurons by abducens internuclear neurons: synaptic specificity vs. intrinsic motoneuron properties. J. Neurophysiol. 78, 1531–1549 (1997). In the context of describing simulations of motor neuron and motor unit recruitment, references 97 and 98 summarize data relevant to these issues and discuss many of the important related conceptual issues.
Porter, J. D., Baker, R. S., Ragusa, R. J. & Brueckner, J. K. Extraocular muscles: basic and clinical aspects of structure and function. Surv. Ophthalmol. 39, 451–484 (1995). A comprehensive review of knowledge about the structural and functional properties of extraocular muscle.
Goldberg, S. J., Wilson, K. E. & Shall, M. S. Summation of extraocular motor unit tensions in the lateral rectus muscle of the cat. Muscle Nerve 20, 1229–1235 (1997).
Goldberg, S. J., Meredith, M. A. & Shall, M. S. Extraocular motor unit and whole-muscle responses in the lateral rectus muscle of the squirrel monkey. J. Neurosci. 18, 10629–10639 (1998).
Goldberg, S. J. & Shall, M. S. Motor units of extraocular muscles: recent findings. Prog. Brain Res. 123, 221–232 (1999). Summaries of what is known about extraoculomotor unit types, how they are distributed within the muscles, and how motor unit forces summate, can be found in references 99–102.
Buttner-Ennever, J. A. (ed.) Neuroanatomy of the Oculomotor System. Reviews of Oculomotor Research Vol. 2 (Elsevier, Amserdam, 1988).
Henn, V., Buttner-Ennever, J. A. & Hepp, K. The primate oculomotor system. I. Motoneurons. Hum. Neurobiol. 1, 77–85 (1982).
Acknowledgements
This work was supported by grants from the National Institutes of Health.
Author information
Authors and Affiliations
Related links
Related links
FURTHER INFORMATION
Encyclopedia of Life Sciences
MIT Encyclopedia of Cognitive Sciences
Glossary
- PLANT
-
A term used in control theory to refer to that which is controlled. In the case of eye movements, the oculomotor plant refers to the globe, extraocular muscles, orbital suspensory tissues and any other passive orbital tissues that influence rotation of the eye.
- CYCLOROTATION
-
Rotations around the anteroposterior axis of the globe are known as cycloductions or cyclorotations.
- ON-DIRECTION
-
The direction of movement associated with the maximal discharge of a neuron.
- NON-COMMUTATIVITY
-
Having the property that the results of a mathematical operation on elements might produce different results depending on the order in which the elements are used.
- LISTING'S LAW OPERATOR
-
A hypothetical neural circuit that performs the computations required to implement Listing's law.
Rights and permissions
About this article
Cite this article
Sparks, D. The brainstem control of saccadic eye movements. Nat Rev Neurosci 3, 952–964 (2002). https://doi.org/10.1038/nrn986
Issue Date:
DOI: https://doi.org/10.1038/nrn986
This article is cited by
-
Concussed patients with visually induced dizziness exhibit increased ocular torsion and vertical vergence during optokinetic gaze-stabilization
Scientific Reports (2023)
-
Brainstem sources of input to the central mesencephalic reticular formation in the macaque
Experimental Brain Research (2023)
-
Volumetric Analysis of Amygdala and Hippocampal Subfields for Infants with Autism
Journal of Autism and Developmental Disorders (2023)
-
Prognostic factors and outcome of pineoblastoma: 10 years single-center experience
Journal of the Egyptian National Cancer Institute (2021)
-
Transforming absolute value to categorical choice in primate superior colliculus during value-based decision making
Nature Communications (2021)