Abstract
Aims
To prospectively evaluate by ultrasound biomicroscopy (UBM) and gonioscopy the anterior chamber angle widening following laser peripheral iridotomy (LPI) in eyes with early chronic primary angle closure glaucoma (CACG).
Methods
A total of 55 eyes of 55 patients with CACG presenting with less than 180° peripheral anterior synechiae (PAS) were enrolled in the study. Angles were assessed by gonioscopy (Shaffer's grading) and UBM, before and 4 weeks after LPI. The angle opening distance at 250 and 500 μm from the scleral spur (AOD 250 and AOD 500) was computed. Results were analysed using the Wilcoxon signed-rank test.
Results
In the quadrant with LPI, the mean gonioscopy grade increased significantly from 0.45 to 1.45 (P<0.001) and the mean AOD 250 and AOD 500 increased from 38.5±25.9 to 83.5±48.4 μm (P<0.001) and 110.2±80.9 to 170.6±83.4 μm (P<0.001), respectively. The angles widened significantly in the opposite quadrant on UBM (AOD 250: 48.8±31.5–82.7±43.9 μm, P<0.001; AOD 500:117.2±65.5–172.2±81.7 μm; P<0.001), but the median gonioscopy grade remained unchanged.
Conclusions
LPI significantly widened the anterior chamber angle in the quadrant with LPI and the quadrant furthest away in patients of CACG with established glaucomatous damage. This change was much better appreciated by the UBM than gonioscopy.
Similar content being viewed by others
Introduction
Primary angle closure glaucoma (PACG) is the most common cause of glaucoma in Asian populations.1 While patients with acute angle closure present in a manner that is rarely missed (with a painful red eye), most people with chronic primary angle closure glaucoma (CACG) present with established glaucomatous damage, and half of them may remain unrecognized.2
It is generally agreed that eyes with occludable iridocorneal angles should undergo laser peripheral iridotomy (LPI).3 In acute primary angle closure, LPI prevents the recurrence of acute attacks, and eliminates the risk of an acute attack in the fellow eye.4 In chronic PACG, where appositional closure gradually leads to peripheral anterior synechiae (PAS) formation, it may reverse the process sufficiently to control the intraocular pressure (IOP).
Recent evidence suggests that the outcome of LPI in Asian races5, 6, 7 is not as favourable as in Caucasian eyes.8, 9, 10 Whether this is due to non-opening of the angle or as a result of progressive trabecular damage following extensive PAS11 remains unclear. Retrospective studies in east Asian eyes have demonstrated a better long-term outcome of LPI in those eyes with <180° synechial closure,5, 6 or when it is carried out at a stage before the glaucomatous optic neuropathy develops.12
LPI works by overcoming the pupillary block; the convex iris flattens, and the anterior chamber angle widens. This is difficult to assess gonioscopically, although it may be possible to appreciate these changes with adequate training and experience. Gonioscopy is a limited by its inability to measure structures in the far periphery of the angle. Being a subjective evaluation, it is also limited by interobserver variation in angle assessment. Objective quantitative measurement of the angle has been greatly enhanced by the ultrasound biomicroscope (UBM),13, 14 which permits reproducible imaging of the anterior chamber angle. However, it must be kept in mind that UBM also has its limitations of artefactually widening the angle by indentation and performing in the supine position.
India seems to have a larger prevalence of PACG compared to the Western population.2, 15 Although the clinical outcome of LPI has been studied in terms of alteration of the disease course,5, 16 there is a paucity of information regarding the morphological alterations of the angle following LPI quantified by the UBM in Indian eyes. This study aimed to quantify, by UBM, the changes in the angle after laser iridotomy in Indian patients with chronic angle closure glaucoma, but with less than 180° of synechial angle closure.
Patients and methods
This was a prospective, non-randomized cross-sectional study. Patients with chronic angle closure glaucoma were enrolled from the Glaucoma Clinic of the Department of Ophthalmology, Postgraduate Institute of Medical Education and Research, Chandigarh, India, between September 2004 and June 2005. The study was cleared from the Institute Ethics Committee, and adhered to the principles enshrined in the Declaration of Helsinki. Informed consent was obtained from all recruited subjects.
Each patient underwent a comprehensive ophthalmic examination including best-corrected visual acuity (BCVA), IOP, slit-lamp biomicroscopy, gonioscopy, and stereoscopic disc evaluation using a 90.0 D lens. This was followed by baseline Standard Achromatic Perimetry (SAP) on the Humphrey's Field Analyzer HFA 750 II (Carl Zeiss-Humphrey Systems, Dublin, CA, USA) using the 24-2 testing protocol by SITA-Standard strategy. The mean deviation (MD) was documented in all patients.
Gonioscopy
Gonioscopy was performed by one of the two investigators (SK, RJ) experienced in using a Sussman four-mirror goniolens. The procedure was carried out in a darkened room with minimum-possible slit-lamp illumination, taking care that the slit beam did not impinge upon the pupil. The angles were graded in all four quadrants numerically according to Shaffer's classification.17 The presence of PAS was looked for on indentation gonioscopy, and the extent noted.
Patients diagnosed as PACG with less than 180° of PAS were included in the study.
Diagnostic criteria included the following: An occludable angle was defined as one in which three-quarters of the posterior pigmented trabecular meshwork was not visible on gonioscopy in the primary position of gaze without indentation. Glaucomatous optic neuropathy was defined as one with cup:disc ratio (CDR) >0.6, or >0.2 CDR asymmetry between the two eyes. A glaucomatous visual field was defined as MD and pattern standard deviation (PSD) values outside 95% confidence interval and a Glaucoma Hemifield Test classified as ‘outside normal limits’.
The diagnosis of PACG was made in the presence of occludable angle as defined above and characteristic optic nerve head changes with corresponding glaucomatous visual field defects reproducible on two field tests.
Patients with any of the following conditions were excluded from the study: advanced glaucoma (CDR >0.8 and/or MD worse than −12 dB); >180° synechial angle closure on indentation gonioscopy; patients with cataract accounting for unaided visual acuity <20/80, who were offered cataract surgery rather than laser iridotomy; primary acute angle closure — painful red eye with drop in visual acuity, raised IOP, closed angles on gonioscopy, and no evidence of secondary angle closure; any history of ocular disease or intraocular surgery, or if they were detected to have any ocular disease during examination, such as diabetic retinopathy or uveitis. If both eyes were eligible, only the right eye was included.
Ultrasound biomicroscopy
UBM uses high-frequency ultrasound (50–100 MHz) to produce images of the anterior segment at high resolution. All examinations were performed with the UBM Model 840® (Paradigm Medical Industries Inc., Salt Lake City, USA), with a 50 MHz transducer probe. To minimize the effect of any anti-glaucoma medication on the angle configuration, it was ensured that all patients had a drug-free period of at least 2 weeks before acquiring the UBM images.
Image acquisition
All UBM measurements were performed by one investigator (SK), who was masked to the patients' diagnosis and gonioscopy finding. After instilling 4% xylocaine drops in the eye, a plastic eyecup was used to gently part the lids and retain a layer of 2% hydroxypropyl methylcellulose coupling agent, with care not to exert pressure on the globe. Scanning was performed in the supine position; the probe was manually moved perpendicular to the structure scanned. Fixation and accommodation was held constant by having the patient fixate with the fellow eye on a ceiling target. The baseline image was acquired before LPI prior to instillation of Pilocarpine drops.
Measurement of the angle
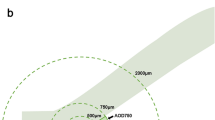
The angle-opening distance (AOD), as defined by Pavlin et al,13 is the distance from corneal endothelium to the anterior iris perpendicular to a line drawn along the trabecular meshwork at a given distance from the scleral spur. This is measured at 250 μm (AOD 250) and 500 μm (AOD 500) from the scleral spur (Figure 1). AOD 250 and AOD 500 were computed for each patient.
Laser peripheral iridotomy
After obtaining an informed consent for the procedure, all patients received 4% Pilocarpine to constrict the pupil and stretch the iris. Apraclonidine 1% was instilled 1 h before the procedure. Nd:YAG LPI was performed in each case using an Abrahams lens and 4–6 mJ energy, with 5–10 shots depending upon the thickness of the iris. All iridotomies were carried out in the superior half, preferably the superonasal quadrant in a crypt of iris, or an area of stromal atrophy. Patients were instructed to instill betamethasone-neomycin drops q.i.d., and Timolol maleate 0.5% twice a day for a week following the procedure.
Patients were followed up at 1, 2 and 4 weeks following LPI. A complete ocular examination was carried out, specifically noting the BCVA, IOP, AC depth, and gonioscopy. Post-LPI, UBM scans were taken after 4 weeks following the procedure, using the same protocol that was used in the pre-LPI evaluation.
Main outcome measures
Data were recorded on prospectively filled forms. Gonioscopy grade and UBM parameters were computed for the angle quadrants with the LPI and the angle quadrant furthest away. The angle parameters and IOP were the main outcome measures studied.
Analysis
Adequacy of sample size
This analysis involved the use of continuous variables in a paired study design. A previous study,18 analysing the change in angle configuration following LPI, reported the mean AOD 500 to have increased by 40 μm with a standard deviation of 70 μm. With these assumptions, the minimum sample size required for a study with a power of 90% at a significance level of 5% was calculated as (1.96+1.28)2 × 702/402=33 patients.
The results were analysed using SPSS for Windows software, Version 10.0, ©SPSS Inc., Chicago, IL, USA. The Wilcoxon signed-rank test was used to compare the mean gonioscopy grades, UBM parameters, and IOP before and after laser iridotomy. The Mann–Whitney U-test was used to analyse the differences between the mean per cent change in gonioscopy and UBM parameters following LPI. The results were considered significant at P< 0.05.
Results
A total of 59 eyes of 59 patients with occludable angles fulfilled the eligibility criteria. Three patients did not return after 4 weeks for the post-LPI UBM measurements, and one patient had irido-ciliary cysts causing secondary angle closure. A total of 55 eyes of 55 patients were included for the final analysis. There were 34 female and 21 male subjects. The mean age of female subjects was less than that of male subjects (49.4±11.1 vs 52.7±11.8 years), although the difference did not reach statistical significance (P=0.315). The mean refractive error was +0.92±0.91 D (Figure 2). Laser iridotomy was carried out in the superonasal quadrant in 44 eyes (80%) and in the superotemporal quadrant in 11 eyes (20%).
The average MD on visual fields was −5.37±2.86 dB. Pre-LPI UBM examination showed anteriorly rotated ciliary processes in 11 of the 55 eyes, suggestive of plateau iris configuration. In the rest, the ciliary sulcus was well defined with normally oriented ciliary processes.
In the quadrant with LPI, the mean gonioscopy grades increased from 0.45 to 1.45 (P<0.001). Post-LPI, the mean AOD 250 and AOD 500 increased from 38.5±25.9 to 83.5±48.4 μm (P<0.001) and 110.2±80.9 to 170.6±83.4 μm (P<0.001), respectively (Table 1 and Figure 3). The difference in the quadrant opposite to the LPI site was more noticeable on UBM than on gonioscopy, where the median gonioscopy grade remained unchanged at 1.00 following iridotomy, although the difference in the mean grade was statistically significant (0.58–0.69; P=0.014). The median AOD 250 and AOD 500 increased by 36 and 45 μm, respectively, and the mean UBM parameters also increased significantly (Table 1 and Figure 3).
The per cent change in angle widening demonstrable on gonioscopy and UBM was compared in both quadrants (Table 2). In the LPI quadrant, there was no significant difference in the mean per cent change in angle widening seen by gonioscopy or UBM (P=0.078). However, in the opposite quadrant, the mean per cent change by UBM was significantly more than that demonstrable by gonioscopy (P<0.001). In both quadrants, the mean per cent change seen in the AOD 500 measurements was less than the change seen on AOD 250 measurements. This difference was significant in the quadrant with the LPI, but not in the opposite quadrant.
The mean IOP decreased from 18.7±3.9 mmHg before LPI to 15.2±2.0 mmHg after LPI (P<0.001). A total of 39 patients were controlled on one or two topical antiglaucoma medications at presentation, whereas the rest of the 16 patients had an IOP >21 mmHg despite treatment. Following LPI, only three patients required medication (latanoprost 0.005%) for IOP control.
Discussion
Angle closure glaucoma is a significant problem in India. The Andhra Pradesh Eye Disease Study (APEDS)2 reported that 2.21% of the population >40.0 years had occludable angles at risk of angle closure and 1.08% had manifest PACG, a large proportion of whom were undiagnosed and untreated. In the Vellore Eye Study (VES),15 manifest PACG was 4.3%. Angle closure glaucoma constituted 46% of all primary adult glaucoma seen in a tertiary-care hospital in north India.19
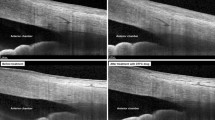
Primary angle closure glaucoma may be considered a disease ‘secondary’ to raised IOP following angle closure, with no inherent susceptibility of the optic nerve head to glaucomatous damage, as has been proposed for normal tension glaucoma or primary open angle glaucoma. This indicates that if the angles could be opened in time, the disease could be prevented, or at least stabilized in cases of established damage. LPI is a treatment modality widely used towards this end. Most studies on the outcome of LPI have concentrated on the disease progression or IOP control, whereas very few have studied the actual effect on the angle. This could be because, until recently, gonioscopy was the only tool available for angle assessment. Its major limitation is the inherent subjectivity of the technique, and the inability to visualize the far recess of the angle. The UBM allows detailed imaging of qualitative changes in anterior segment morphology following LPI like the flattening of the convex iris and widening of the anterior chamber angle (Figure 4). More importantly, it has enabled clinicians to measure the angle width quantitatively with a greater degree of objectivity.
The present study included eyes with CACG and less than 180° PAS. The mean defect on visual fields was less than −6.0 dB in the majority of patients, which falls into the category of ‘early’ glaucoma as defined by Hodapp et al.20 By the same corollary, our study group may be considered ‘early’ primary CACG.
Asian eyes have been known to progress to chronic PACG despite a patent iridotomy, and these eyes require additional medication or surgery for IOP control.5, 6, 7 Aung6 and Allsagoff et al7 demonstrated that the LPI alone was successful in lowering the IOP only in those patients with less than 180° of PAS. Ang et al21 reported 80 fellow eyes of acute primary angle closure glaucoma who underwent an LPI, of whom 88.8% were successfully treated with the iridotomy alone without the need for additional glaucoma treatment in the long term. However, a small proportion of fellow eyes in their study did experience rise in IOP within the first year despite a patent LPI. Nolan et al12 found that iridotomy was more likely to control the disease process in eyes with no glaucomatous optic neuropathy. Our idea was to limit our study patients to those where the effect of LPI on the angle was not likely to be confounded by other factors such as extensive PAS, and lens thickening because of cataract. We also investigated the extent of the effect of LPI, by comparing the changes in angle width in the quadrant with the LPI as well as in the quadrant furthest away. Although this study was investigating the morphological change in angle width following LPI, we did include IOP as an outcome measure, as it can be considered an indirect marker for functional angle opening.
Our study demonstrated that LPI was effective in widening the angle even in patients with established early glaucomatous optic nerve damage (both in the quadrant with the LPI and in the quadrant opposite the site), but the change was far more evident by UBM than gonioscopy, especially in the opposite quadrant. Here, the mean per cent gonioscopy change was significantly less than the mean per cent change in the UBM measurements. Even in the quadrant with the LPI, although the difference was not statistically significant, the per cent gonioscopy change was less than the per cent change in UBM measurements. In both the quadrants, the AOD 500 measurements changed less than the AOD 250 measurements. This means that the root of the iris open more than the part peripheral to it, and AOD 250 may be considered a more useful parameter in evaluating angle widening after LPI.
One interesting finding was that the mean gonioscopy grade of the LPI quadrant increased by 1, but the opposite quadrant increased by only 0.11. One probable explanation could be that the constant percolation of aqueous through the LPI keeps that quadrant more open compared to the quadrant opposite the LPI site. This angle opening was corroborated by a significant decrease in IOP after the procedure. Fifty-two of the 55 patients could be controlled without medication following the intervention, compared to none prior to it.
Few other studies have reported UBM changes following LPI, of which none is in Indian eyes. Maraffa et al22 studied a group of undefined (stage of glaucoma or the extent of PAS not specified) PACG patients, and found that the anterior chamber angle widened following LPI, although they used lines drawn between the peripheral iris and cornea as the boundaries of the angle. The angle opening thus measured is prone to measurement errors because of anatomic variations in iris configuration.
Caronia et al23 demonstrated an increase in the AOD and the lens–iris contact following LPI, with a flattening of the convex iris configuration. Gazzard et al18 showed widening of the anterior chamber angle following LPI with no change in the central anterior chamber depth. Yoon et al24 compared the UBM change in angle morphology following LPI and trabeculectomy, and demonstrated significant increases in AODs after both procedures.
The changes occurring in the angle configuration following laser iridotomy in Indian eyes have not been quantified by the UBM before. One Indian study of anterior segment features of PACG eyes imaged by the UBM included those eyes that had undergone LPI, but no baseline imaging had been carried out.25 None of the other studies have compared the angle opening at the site of the LPI with the rest of the angle. Although the benefits of LPI are well established, the appropriate timing of the intervention in CACG is still uncertain. In our study, the presence of established glaucoma per se did not appear to result in a less favourable outcome. (However, as extensive synechial closure is likely to result in more uncontrolled IOP, the stage of glaucoma may be considered to be an indirect indicator of the extent of PAS formation.)
Patients with chronic angle closure glaucoma have been reported to have uncontrolled IOP despite a patent iridotomy, owing to extensive synechiae formation and trabecular damage.11 A timely LPI may retard these changes and effectively control IOP if performed early in the disease process. However, keeping in mind the poorer long-term effects of LPI in Asian races, a longitudinal study of these patients is required to analyse what exactly happens in the long term to an angle that has been successfully opened by LPI.
References
Quigley HA . Number of people with glaucoma worldwide. Br J Ophthalmol 1996; 80: 389–393.
Dandona L, Dandona R, Mandal P, Srinivas P, John RK, McCarty CA et al. Angle closure glaucoma in an urban population in southern India. The Andhra Pradesh Eye Disease Study. Ophthalmology 2000; 107: 1710–1716.
Slamovits T, Dutton JJ . Should patients with anatomically narrow angles have prophylactic iridectomy? Surv Ophthalmol 1996; 41: 31–36.
Ritch R . Assessing the treatment of angle closure. Ophthalmology 2003; 110 (10): 1867–1868.
Sihota R, Sood A, Gupta V, Dada T, Agarwal HC . A prospective long-term study of primary chronic angle closure glaucoma. Acta Ophthalmol Scand 2004; 82: 209–213.
Aung T, Ang LP, Chan SP, Chew PTK . Acute primary angle closure: long term IOP outcome in Asian eyes. Am J Ophthalmol 2001; 131: 7–12.
Allsagoff Z, Aung T, Ang LPK, Chew PTK . Long-term clinical course of primary angle closure glaucoma in an Asian population. Ophthalmology 2000; 107: 2300–2304.
Salmon JF . Long-term intraocular pressure control after Nd: YAG laser iridotomy in chromic angle closure glaucoma. J Glaucoma 1993; 2: 291–296.
Robin AL, Pollock IP . Argon laser peripheral iridotomy in the treatment of primary angle closure glaucoma: long-term follow-up. Arch Ophthalmol 1982; 100: 919–923.
Fleck BW, Wright E, Fairley EA . A randomized prospective comparison of operative peripheral iridectomy and Nd:YAG laser iridotomy treatment of acute angle closure glaucoma: 3 year visual acuity and IOP control outcome. Br J Ophthalmol 1997; 81: 884–888.
Sihota R, Lakshmaiah NC, Walia KB, Sen S, Jaias F, Jayalakshmi P . Ultrastructural changes in the trabecular meshwork of eyes with acute and chronic angle closure glaucoma. Inv Ophthalmol Vis Sci 1999; 40: S669.
Nolan WP, Foster PJ, Devereux JG, Uranchimeg D, Johnson GJ, Baasanhu J . Yag laser iridotomy treatment for primary angle closure in east Asian eyes. Br J Ophthalmol 2000; 84: 1255–1259.
Pavlin CJ, Harasiewicz K, Sherar MD, Foster FS . Clinical use of ultrasound biomicroscopy. Ophthalmology 1991; 98: 287–295.
Pavlin CJ, Harasiewicz K, Eng P, Foster FS . Ultrasound biomicroscopy of the anterior segment structures in normal and glaucomatous eyes. Am J Ophthalmol 1992; 113: 381–389.
Jacob A, Thomas R, Koshi SP, Braganza A, Muliyil J . Prevalence of glaucoma in an urban South Indian population. Ind J Ophthalmol 1998; 46: 81–86.
Thomas R, Arun T, Muliyil J, George R . Outcome of laser peripheral iridotomy in chronic primary angle closure. Ophthalmic Surg Lasers 1999; 30: 547–553.
Shaffer RN . Gonioscopy, ophthalmoscopy and Perimetry. Trans Am Acad Ophthlamol Otolaryngol 1960; 64: 112–116.
Gazzard G, Friedman DS, Devereux JG, Chew P, Seah SK . A prospective ultrasound biomicroscopy evaluation of changes in anterior segment morphology after laser iridotomy in Asian eyes. Ophthalmology 2003; 110 (3): 630–638.
Sihota R, Agarwal HC . Profile of the subtypes of angle closure glaucoma in a tertiary hospital in north India. Indian J Ophthalmol 1998; 46 (1): 25–29.
Hodapp E, Parrish RK, Anderson DR . Clinical decisions in Glaucoma. Mosby: St Louis, 1999; 52–54.
Ang LP, Aung T, Chew PT . Acute primary angle closure in an Asian population: long term outcome of te fellow eye after prophylactic laser peripheral iridotomy. Ophthalmology 2000; 107: 2092–2096.
Maraffa M, Marchini G, Pagiarusco A . Ultrasound biomicroscopy and corneal endothelium in Nd:Yag laser iridotomy. Ophthalmic Surg Lasers 1995; 26: 519–523.
Caronia RM, Liebmann JM, Stegman Z, Sokol J, Ritch R . Increase in iris–lens contact after laser iridotomy for pupillary block angle closure. Am J Ophthalmol 1996; 122 (1): 53–57.
Yoon KC, Won KLD, Cho HJ, Yuong KJ . Ultrasound biomicroscopic changes after laser iridotomy or trabeculectomy in angle closure glaucoma. Korean J Ophthalmol 2004; 18: 9–14.
Garudadri C, Chelerkar MS, Nutheti R . An ultrasound biomicroscopic study of the anterior segment in Indian eyes with primary angle closure glaucoma. J Glaucoma 2002; 11: 502–507.
Author information
Authors and Affiliations
Corresponding author
Additional information
No author has any proprietary interest in any of the products mentioned in this paper.
Rights and permissions
About this article
Cite this article
Kaushik, S., Kumar, S., Jain, R. et al. Ultrasound biomicroscopic quantification of the change in anterior chamber angle following laser peripheral iridotomy in early chronic primary angle closure glaucoma. Eye 21, 735–741 (2007). https://doi.org/10.1038/sj.eye.6702317
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6702317
Keywords
This article is cited by
-
Optical coherence tomography analysis of anterior segment parameters before and after laser peripheral iridotomy in primary angle-closure suspects by using CASIA2
BMC Ophthalmology (2022)
-
A prospective ultrasound biomicroscopy evaluation of changes in anterior segment morphology following laser iridotomy in European eyes
Eye (2009)