Abstract
Aim
To report the safety and efficacy of intraoperative mitomycin (MMC) augmentation of combined phacoemulsification and deep sclerectomy (PDS).
Methods
Retrospective, non-randomized, comparative, interventional case series of 119 eyes (63 with and 56 without MMC augmentation) of 119 patients who had PDS between September 2001 and April 2004.
Results
The mean follow-up was 23 months (range 12–41 months). There were no differences in the baseline characteristics of the two groups except that patients from the phacoemulsification and deep sclerectomy with mitomycin C (PDS-MMC) group were on average, younger by 3 years (P=0.01). Two years after surgery, the probability of maintaining an IOP below 19 and 15 mmHg without glaucoma medications or needle revision was 76 and 62% in the PDS-MMC group and 62 and 45% in the PDS-no MMC group (P=0.02 and 0.04, respectively). Nd:YAG laser goniopuncture was performed in 71.4% of eyes in the PDS-no MMC and 61.9% of the PD-MMC group (P=0.33). Needle revision was performed in 21.4% of the PDS-no MMC and 17.4% of the PDS-MMC group (P=0.65). Ten patients (8.4%) lost more than two lines of Snellen's visual acuity during follow-up, with no difference between the groups. There were few serious complications related to MMC use (hypotony in one eye after laser goniopuncture). The overall incidence of transconjunctival oozing in the PDS-MMC group was 9.5% compared with 5.4% in the PDS-no MMC group.
Conclusion
This study demonstrates that augmentation of PDS with MMC is safe. MMC augmentation appears to increase the probability of achieving lower target intraocular pressures after combined PDS.
Similar content being viewed by others
Introduction
Deep sclerectomy (DS), an initially non-penetrating glaucoma procedure (NPGS), has been suggested as an alternative to trabeculectomy. IOP lowering with DS and postoperative laser goniopuncture has been shown to be comparable to trabeculectomy with a lower incidence of complications in the immediate postoperative period, according to some reports.1, 2, 3, 4 In the presence of significant cataract with glaucoma, combined phacoemulsification with trabeculectomy is commonly performed.5 Phacoemulsification combined with deep sclerectomy (PDS) has been shown to be as effective as phacoemulsification with trabeculectomy (PT) in lowering IOP.3, 6
Mitomycin C (MMC) augmentation increases the IOP lowering effect of glaucoma surgery, but there are concerns regarding a higher risk of later complications.7, 8 We have reported previously that MMC-augmented PDS appears to be at least as efficacious as MMC-augmented PT, with low complication rates for both procedures.9 However, there are no studies directly comparing MMC-augmented PDS to non-augmented PDS.
The aim of this study was to investigate the comparative safety and efficacy of combined phacoemulsification and deep sclerectomy with mitomycin C (PDS-MMC) and without (PDS-no MMC) in lowering intraocular pressure.
Patients and methods
This study is a retrospective, comparative, and non-randomized case series. Consecutive patients undergoing combined phaco-deep sclerectomy (phaco-DS), with or without MMC, between September 2001 and April 2004 were included. The total number of eligible patients was 121 and the eye operated first was included. Two patients died soon after surgery and hence were excluded. Five patients (4.2%) with follow-ups of more than a year died during the study period. Data were entered at the time of patient visit to the office on a correlational Microsoft Access™ database as part of an ongoing audit on all glaucoma surgeries in our department.
All procedures were performed or supervised closely by one surgeon (NA) using a standardized technique described previously.9 Briefly, this consisted of a standard superior DS followed by temporal clear corneal phacoemulsification. A diamond blade was used to outline a trapezoid-shaped superficial limbal-based scleral flap (5 mm at the limbus and 3 mm at the apex). This was dissected with a number 11 beaver blade down to approximately one-third scleral thickness. The dissection was carried 1–2 mm into clear cornea. The deep scleral flap was delineated 1 mm within the edges of the superficial flap. The edges were deepened till the choroid was visible at the posterior edge and Schlemm's canal incised at the lateral edges. The deep flap was then dissected in the plane of the scleral spur, de-roofing Schlemm's canal, and dissection was continued 1–2 mm into clear cornea before being excision of the inner flap. The juxta-canalicular trabecular meshwork (JXT) was then peeled using capsulorrhexis forceps till adequate flow was observed. The superficial scleral flap was sutured back loosely with two 10/0 nylon sutures. In later cases, a 3.5 mm reticulated hyaluronic acid implant (SKGEL™, Corneal Inc, France) was placed into the scleral bed as a spacer device before flap suturing. The conjunctiva was loosely secured to clear cornea with two 10/0 nylon radial interrupted sutures. An Acrysof™ (Alcon Labs, USA) intraocular lens implants were used in all cases. MMC augmentation was selective.
Indications for augmented surgery included eyes at a higher risk for failure such as Afro-Caribbean race, previous intraocular surgery, long-term medications, and a low target IOP. MMC augmentation was avoided in elderly patients and in eyes with fragile conjunctiva and minimal subconjunctival tissues. A fornix-based conjunctival flap was made in all cases. MMC (0.2 mg/ml for 2 min for most cases) applied before scleral flap dissection. In earlier cases, MMC-soaked cellulose sponge pieces (Cellulose Spears, Eyetec Ophthalmic Products, Altomed Ltd, UK) were applied anteriorly on the area of scleral flap dissection. The technique for MMC application was changed during the study, as suggested by Wells et al,10 to avoid cystic filtration blebs MMC soaked in polyvinyl alcohol sponge pieces (PVA Spears, Eyetec Ophthalmic Products, Altomed Ltd, UK) were used to avoid fragmentation.11, 12 These were applied more posteriorly, avoiding area of scleral flap and the conjunctival edge was protected by Khaw's 5 mm conjunctival clamp (Duckworth and Kent, UK).
Postoperative management consisted of topical steroid–antibiotic combination for 1 week followed by topical steroids for at least another 2 months. A careful slit-lamp examination under high magnification (× 16) for subconjunctival filtration was performed at each postoperative visit by one observer (NA). The area of bleb avascularity, if any, was measured and these avascular areas were checked for transconjunctival oozing.13, 14 Subconjunctival betamethasone 0.1 ml (0.4 mg) was injected in the early postoperative period if signs of bleb failure were observed. Nd:YAG laser goniopuncture was delayed for at least a month after surgery and was performed if IOP increased over target range. The technique for goniopuncture is described elsewhere.15 Needle revision with subconjunctival 5-fluorouracil (5-FU) (5 mg) and in later cases, MMC (0.02 mg), was performed in the office if laser goniopuncture failed to lower IOP. Gonioscopy was performed regularly to check for iris incarceration in perforations or laser punctures of the trabeculo-Descemet's membrane (TDM) window.
Statistical analysis
Analysis was performed on an intention to treat basis. Statistica6 (Statsoft Inc, USA) was used for statistical analysis. Three criteria for success were used for Kaplan–Meier survival analysis. These included:
-
1
IOP between 5–19 mmHg without additional glaucoma medications or any further surgical procedure including needle revisions to lower IOP. Laser goniopuncture was not considered as failure. Therefore, one IOP measurement more than 18 mmHg, before laser goniopuncture was permitted.
-
2
IOP to be maintained between 5–15 mmHg without additional medications or further procedure to lower IOP except laser goniopuncture.
-
3
Normal tension glaucoma–IOP to be maintained 30% below the baseline. Baseline IOP was the average of four Goldmann Applanation Tonometer measurements in a morning, without the patient taking any glaucoma medication. In all cases, the target IOP for these eyes was less than 15 mmHg and a 30% drop from baseline.
A Cox regression model was used to determine the effect of various factors on the survival curves. Comparisons between the two groups were obtained by log-rank test. Dichotomous variables were analysed using χ2 tests with Yates's correction and the Fisher's exact tests. Analyses of variance (ANOVA) were used for continuous variables. Non-parametric data were analysed by the Mann–Whitney U tests, Wilcoxin matched pairs test for paired data, and Kruskal–Wallis ANOVA for. All tests were statistical two-tailed and P-values less than 0.05 were considered significant.
Results
Patient demographics and preoperative characteristics are shown in Table 1. There were 63 eyes in the PDS-MMC group and 56 eyes in the PDS-no MMC group. There was no significant difference between the two groups except that patients were younger in the MMC group. The preoperative IOP and number of topical medications were marginally higher in the no-MMC group, but this was not statistically significant. The PDS-no MMC had slightly higher preoperative IOPs than the PDS-MMC group, but this difference was not significant.
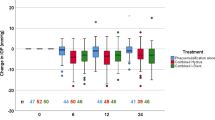
IOP changes after surgery for the two groups are shown in Figure 1. The IOP for the PDS-MMC group was lower for all time intervals. It was significantly lower (P<0.05) in the following time intervals; weeks 1–2, weeks 3–6, months 2–3, and months 8–10. The probabilities (with 95% confidence intervals) of maintaining IOP below 19 and 15 mmHg for the PDS-MMC and PDS-no MMC groups respectively was 85.7% (77.1–94.4%) and 71.4% (59.6–83.3%) at 1 year (P=0.02) and 75.9% (62.8–89%) and 59.3% (45.4–73.2%) at 2 years (P=0.04). Figures 2 and 3 show Kaplan–Meier survival curves for IOP below 19 and 15 mmHg, respectively. A Cox regression analysis model for possible factors affecting survival (IOP less than 15 mmHg) showed a significant effect (P=0.02) of MMC application and of postoperative iris incarceration or obstruction of the TDM window by iris synechiae (Table 2).
Surgical complications are shown in Table 3 and subsequent additional procedures in Table 4. There was one case of hypotony after laser goniopuncture, which did not resolve despite intra-cameral injection of high-molecular-weight hyaluronic acid (Healon GV™) and required drainage of the associated choroidal detachment. Regular gonioscopy in all eyes by one observer (NA) showed that iris incarceration in a perforation or laser puncture site or synechiae in the region of the TDM window was seen in 16% of all eyes. The distribution of conjunctival filtration bleb avascularity, cystic areas, and transconjunctival oozing are shown in Table 5. There was no significant difference in the frequency of bleb avascularity between the two groups (P=0.08). No encapsulated blebs were seen. No delayed bleb leaks were observed in either group.
Visual acuity changes for all eyes after PDS are depicted in Figure 4 and after excluding ocular comorbidity, no differences were observed in the improvement of visual acuity between the two groups (P=0.47). A decrease of two Snellen chart lines from preoperative VA was observed in 10 eyes (8.4%). The causes were age-related macular degeneration in two eyes each of both groups, idiopathic epiretinal membranes in four eyes of the PDS-no MMC group, central retinal vein occlusion in one eye of the PDS-MMC group, and severe ocular surface disease in one eye of PDS-no MMC group.
There was a significant decrease in the mean number of medications required to control IOP from 1.8±1 before 0.2±0.5 after surgery (P=0.00). At the last follow-up, 13 eyes of phaco-DS (23.2%) and seven eyes (11.1%) of the PDS-MMC group were on medications to lower IOP (P=0.09). The PDS-no MMC group were on a significantly higher number of medications for lowering IOP at the following time intervals: months 24–29 (P=0.02) and months 30–35 (P=0.03).
Discussion
The MMC and no-MMC groups in this study were evenly matched, except for the slightly older mean age in the no-MMC group. This may suggest a selection bias as younger patients were more likely to receive MMC, being at higher risk for failure. Conversely a difference of 3 years between the mean ages of patients in the two groups is not likely to be clinically significant. Due to the retrospective uncontrolled design of the paper, there were some changes in surgical technique, principally the use of spacer devices. However Cox's regression analyses did not show any effect of using the spacer device.
The IOP criteria for success in our study were based on the outcomes of the Advanced Glaucoma Intervention Study (AGIS) and Collaborative Normotensive Glaucoma Treatment (NTG) trials. The AGIS post hoc analyses have demonstrated the importance of maintaining IOP below 18 mmHg in patients with established glaucomatous field loss. The group with the least chances of progression in this trial had an average IOP of 12.3 mmHg over a period of 5 years.16 The NTG trial demonstrated a slower visual field progression if IOPs were reduced 30% from baseline.17 The average target IOP in our study was quite low, at 15.15 mmHg. Setting low target IOP levels has implications as evidenced by a relatively high frequency of postoperative manipulations in this study. Nd:YAG laser goniopuncture was performed on 66% of eyes in the follow-up period and needle revision was performed on 20% as the goniopuncture had failed to lower IOP to our target range. Therefore, the DS part of the PDS procedure, as performed in this report, can be more aptly described as an ‘initially’ non-penetrating procedure. Studies with longer follow-ups on DS also show that 40–50% eventually undergo goniopuncture.18, 19
One of the important findings of this study was the high incidence (16%) of iris incarceration in perforation or puncture site and peripheral anterior synechiae in the region of the TDM window and their significant association with failure of PDS (hazard ratio 2.85). IOP rise after iris prolapse into surgical site or iris synechia after laser goniopuncture site has been reported previously.20, 21, 22 The frequency of this complication was 11.9% in a report of gonioscopy after DS23 and 9.7% in a large, 6-year retrospective study on DS.19 Iris incarceration or touch after needle revision in eyes, where laser goniopuncture did not lower IOP, was observed in five eyes (4.1%). Needle revision for failing blebs after DS or PDS has not been reported in these studies and this may account for the slightly higher incidence of iris incarceration in the present study. There were relatively few serious complications in either group.
Our results compare favourably to previous published literature on PDS as well as PT despite more stringent criteria for success. We were able to find only one published prospective trial comparing PDS to PT on a Medline search. Gianoli et al6 reported that the probability of maintaining IOP<21 mmHg without medication at 18 months was in 49% in PDS and 52% after PT. The PDS group had a lower rate of postoperative inflammation and hyphema Cillino et al prospectively compared DS and PDS to trabeculectomy and PT. No additional postoperative measures like Nd:YAG laser goniopuncture, laser suturelysis, and antimetabolites were performed. They were unable to show a difference in IOPs or survival curves between the four groups but concluded that if low target IOPs were required, PT was the procedure of choice.3 A retrospective, comparative report by one of the authors of the current study showed no difference between the outcomes of PDS and PT, both augmented by MMC. The PT group had a slightly higher incidence of delayed bleb leaks.9 The term ‘partial’ success (IOP below the predetermined level with additional glaucoma medications) has been deliberately avoided in this study as it detracts from assessing the true efficacy of the surgical procedure.
The role of MMC in increasing IOP success rates after primary trabeculectomy is now well established.24 The evidence for a similar role for MMC in primary DS is increasing, although by no means beyond doubt.15, 25, 26 A recent report suggests no difference in IOP and safety outcomes between MMC-augmented primary trabeculectomy and DS.27 A review of english language literature on combined cataract and glaucoma surgery (PT) concluded that the ‘preponderance of evidence from the literature suggests a small (2–4 mmHg) benefit from the use of MMC, but not 5-FU, in combined cataract and glaucoma surgery (evidence grade B or moderate).28 In this study, MMC augmentation of PDS also resulted in a small, 2–3 mmHg benefit. Cohen et al compared PT with and without MMC in a placebo-controlled, double-masked trial. They showed a significantly greater reduction in mean IOP in the MMC group compared with the non-MMC group at 12 months (7.05–7.65 vs 2.62–3.84 mmHg). They found a slightly higher incidence of wound leak and hypotony in the MMC group, although the difference was not statistically significant.29 Shin et al30 found MMC to be beneficial in primary PT in eyes with particular risk factors–black race, diabetes mellitus, IOP>20 mmHg on maximal tolerated medication, or at least two topical medications preoperatively Although they found no statistically significant difference in the complication rate in the two groups, there were two cases of choroidal detachment, one corneal epithelial defect, and one Staphylococcus epidermidis endophthalmitis in the MMC group compared with none in the control group. Both Shin and co-workers and Cohen and co-workers used higher concentrations of MMC than in the current study. Cohen et al29 applied it for 2.5 min at the completion of PT Shin et al31 applied MMC subconjunctivally at the end of scleral flap dissection but randomized the duration of application to 1, 3, and 5 min. They did not find a statistically significant difference in the outcome between the groups.
One of the concerns of MMC augmentation after trabeculectomy has been the high incidence of avascular, thin-walled blebs and delayed wound leaks, hypotony, blebitis, and endopthalmitis.7, 8, 13, 32 The risk of developing avascular blebs is also quite high after MMC-augmented DS and PDS.14, 15 We changed the technique of MMC application, as suggested by Wells et al.10 Conjunctival filtration blebs after the change seem to be less likely to develop avascular cystic areas or exhibit transconjunctival oozing (Table 5), with no effect on actuarial success (P=0.54 for IOP<15 mmHg).
In summary, this study suggests that MMC augmentation of PDS appears to be a safe and effective surgical technique, with an increased probability of lowering intraocular pressures to target levels. The non-randomized, retrospective design, and variation in surgical technique are major limitations of this study. A randomized prospective trial is needed to clearly establish that MMC augmentation of PDS results in lower IOPs.
References
Ambresin A, Shaarawy T, Mermoud A . Deep sclerectomy with collagen implant in one eye compared with trabeculectomy in the other eye of the same patient. J Glaucoma 2002; 11: 214–220.
Mermoud A, Schnyder CC . Nonpenetrating filtering surgery in glaucoma. Curr Opin Ophthalmol 2000; 11: 151–157.
Cillino S, Pace FD, Casuccio A, Calvaruso L, Morreale D, Vadala M et al. Deep sclerectomy vs punch trabeculectomy with or without phacoemulsification: a randomized clinical trial. J Glaucoma 2004; 13: 500–506.
El Sayyad F, Helal M, El Kholify H, Khalil M, El Maghraby A . Nonpenetrating deep sclerectomy vs trabeculectomy in bilateral primary open-angle glaucoma. Ophthalmology 2000; 107: 1671–1674.
Friedman DS, Jampel HD, Lubomski LH, Kempen JH, Quigley H, Congdon N et al. Surgical strategies for coexisting glaucoma and cataract: an evidence-based update. Ophthalmology 2002; 109: 1902–1913.
Gianoli F, Schnyder CC, Bovey E, Mermoud A . Combined surgery for cataract and glaucoma: phacoemulsification and deep sclerectomy compared with phacoemulsification and trabeculectomy. J Cataract Refract Surg 1999; 25: 340–346.
Bindlish R, Condon GP, Schlosser JD, D'Antonio J, Lauer KB, Lehrer R . Efficacy and safety of mitomycin-C in primary trabeculectomy: five-year follow-up. Ophthalmology 2002; 109: 1336–1341.
DeBry PW, Perkins TW, Heatley G, Kaufman P, Brumback LC . Incidence of late-onset bleb-related complications following trabeculectomy with mitomycin. Arch Ophthalmol 2002; 120: 297–300.
Funnell CL, Clowes M, Anand N . Combined cataract and glaucoma surgery with mitomycin C: phacoemulsification-trabeculectomy compared to phacoemulsification-deep sclerectomy. Br J Ophthalmol 2005; 89: 694–698.
Wells AP, Cordeiro MF, Bunce C, Khaw PT . Cystic bleb formation and related complications in limbus- vs fornix-based conjunctival flaps in pediatric and young adult trabeculectomy with mitomycin C. Ophthalmology 2003; 110: 2192–2197.
Shin DH, Tsai CS, Kupin TH, Olivier MM . Retained cellulose sponge after trabeculectomy with adjunctive subconjunctival mitomycin C. Am J Ophthalmol 1994; 118: 111–112.
Poole TRG, Gillespie IH, Knee G, Whitworth J . Microscopic fragmentation of ophthalmic surgical sponge spears used for delivery of antiproliferative agents in glaucoma filtering surgery. Br J Ophthalmol 2002; 86: 1448–1449.
Matsuo H, Tomidokoro A, Suzuki Y, Shirato S, Araie M . Late-onset transconjunctival oozing and point leak of aqueous humor from filtering bleb after trabeculectomy. Am J Ophthalmol 2002; 133: 456–462.
Anand N, Arora S, Clowes M . Mitomycin C augmented glaucoma surgery: evolution of filtering bleb avascularity, transconjunctival oozing, and leaks. Br J Ophthalmol 2006; 90: 175–180.
Anand N, Atherley C . Deep sclerectomy augmented with mitomycin C. Eye 2005; 19: 442–450.
The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS investigators. Am J Ophthalmol 2000; 130: 429–440.
Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol 1998; 126: 498–505.
Mermoud A, Karlen ME, Schnyder CC, Sickenberg M, Chiou AG, Hediguer SE et al. Nd:Yag goniopuncture after deep sclerectomy with collagen implant. Ophthalmic Surg Lasers 1999; 30: 120–125.
Lachkar Y, Neverauskiene J, Jeanteur-Lunel MN, Gracies H, Berkani M, Ecoffet M et al. Nonpenetrating deep sclerectomy: a 6-year retrospective study. Eur J Ophthalmol 2004; 14: 26–36.
Hyams M, Geyer O . Iris prolapse at the surgical site: a late complication of nonpenetrating deep sclerectomy. Ophthalmic Surg Lasers Imaging 2003; 34: 132–135.
Kim CY, Hong YJ, Seong GJ, Koh HJ, Kim SS . Iris synechia after laser goniopuncture in a patient having deep sclerectomy with a collagen implant. J Cataract Refract Surg 2002; 28: 900–902.
Vuori ML . Complications of Neodymium:YAG laser goniopuncture after deep sclerectomy. Acta Ophthalmol Scand 2003; 81: 573–576.
Vuori ML . Gonioscopic view of the trabeculo-Descemet's membrane after deep sclerectomy. Acta Ophthalmol Scand 2004; 82: 154–157.
Wilkins M, Indar A, Wormald R . Intra-operative mitomycin C for glaucoma surgery. Cochrane Database Syst Rev 2005; CD002897.
Kozobolis VP, Christodoulakis EV, Tzanakis N, Zacharopoulos I, Pallikaris IG . Primary deep sclerectomy vs primary deep sclerectomy with the use of mitomycin C in primary open-angle glaucoma. J Glaucoma 2002; 11: 287–293.
Neudorfer M, Sadetzki S, Anisimova S, Geyer O . Nonpenetrating deep sclerectomy with the use of adjunctive mitomycin C. Ophthalmic Surg Lasers Imaging 2004; 35: 6–12.
Cillino S, Di PF, Casuccio A, Lodato G . Deep sclerectomy vs punch trabeculectomy: effect of low-dosage mitomycin C. Ophthalmologica 2005; 219: 281–286.
Jampel HD, Friedman DS, Lubomski LH, Kempen JH, Quigley H, Congdon N et al. Effect of technique on intraocular pressure after combined cataract and glaucoma surgery: an evidence-based review. Ophthalmology 2002; 109: 2215–2224.
Cohen JS, Greff LJ, Novack GD, Wind BE . A placebo-controlled, double-masked evaluation of mitomycin C in combined glaucoma and cataract procedures. Ophthalmology 1996; 103: 1934–1942.
Shin DH, Kim YY, Sheth N, Ren J, Shah M, Kim C et al. The role of adjunctive mitomycin C in secondary glaucoma triple procedure as compared to primary glaucoma triple procedure. Ophthalmology 1998; 105: 740–745.
Shin DH, Ren J, Juzych MS, Hughes BA, Kim C, Song MS et al. Primary glaucoma triple procedure in patients with primary open-angle glaucoma: the effect of mitomycin C in patients with and without prognostic factors for filtration failure. Am J Ophthalmol 1998; 125: 346–352.
Hu CY, Matsuo H, Tomita G, Suzuki Y, Araie M, Shirato S, Tanaka S . Clinical characteristics and leakage of functioning blebs after trabeculectomy with mitomycin-C in primary glaucoma patients. Ophthalmology 2003; 110: 345–352.
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented as a scientific poster at the 2005 annual meeting of American Academy of Ophthalmology
Rights and permissions
About this article
Cite this article
Anand, S., Anand, N. Combined phacoemulsification and deep sclerectomy (PDS) with intraoperative mitomycin C (MMC) augmentation. Eye 22, 1040–1049 (2008). https://doi.org/10.1038/sj.eye.6702833
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6702833