Abstract
Aims
To pilot the use of the Cataract National Dataset (CND) using multi-centre data from Electronic Patient Record (EPR) systems and to demonstrate the ability of the CND to deliver certain of its intended benefits, including detailed preoperative profiling of cataract surgery patients and updating of benchmark standards of care in the NHS and beyond.
Methods
NHS departments using EPR systems to collect a minimum preoperative, anaesthetic, operative and postoperative data set, the CND, were invited to submit data, which were remotely extracted, anonymised, assessed for conformity and completeness, and analysed.
Results
Four-hundred and six surgeons from 12 NHS Trusts submitted data on 55 567 cataract operations between November 2001 and July 2006 (86% from January 2004). Mean age (SD) was 75.4 (10.4) years, 62.0% female. Surgery was for first eyes in 58.5%, under local anaesthesia in 95.5% and by phacoemulsification in 99.7%. Trainees performed 33.9% of operations. Preoperative visual acuity (VA) was 6/12 or better in 42.9% eyes overall, in 35.3% first eyes and in 55.3% second eyes. Complication rates included the following: posterior capsule rupture and/or vitreous loss of 1.92%, simple zonule dialysis of 0.46% and retained lens fragments of 0.18%. Postoperative VA of 6/12 or better (and 6/6 or better) was achieved for 91.0% (45.9%) of all eyes, 94.7% (51.0%) of eyes with no co-pathologies and 79.9% (30.2%) of eyes with one or more co-pathologies respectively.
Conclusions
The CND is fit for purpose, is able to deliver useful benefits and can be collected as part of routine clinical care via EPR systems. This survey confirms shifts in practice since the 1997–1998 UK National Survey with full conversion to phacoemulsification, better preoperative acuity, a halving of the surgical ‘index’ benchmark complication of posterior capsule rupture and/or vitreous loss, and improved VA outcomes.
Similar content being viewed by others
Introduction
Cataract surgery is the most commonly performed operation in the NHS. Over the past decade and a half the number of cataract operations performed annually has trebled, with an estimated 105 000 in the United Kingdom in 1990,1 and for England alone 153 000 in 1997–1998,2 306 000 in 2004–2005 with a slight drop in 2005–2006 to 287 0003 (Figure 1).
Looking further back, a 10-fold increase occurred in England between 1968 and 20034 with notable regional variations. Despite this prodigious throughput, few ophthalmic departments can provide robust evidence of visual impairment or co-pathology preoperatively, operative complications, or postoperative clinical outcomes for their patients. The Royal College of Ophthalmologists has previously organised and hosted two Department of Health funded National Cataract Surgery Surveys in 1990 and 1997–1998, to examine variations in the organisation of cataract surgery services and clinical outcomes.1, 5, 6, 7, 8 Both national surveys were, in part, designed to encourage all United Kingdom consultants to perform regular audit of their cataract surgery and to provide benchmark standards by which surgeons and departments could judge their performance. Our previous paper described the substantial improvements in productivity of NHS cataract services brought about by major organisational changes promoted by the ‘Action on Cataracts’ programme and the almost universal switch to day case, local anaesthetic, phacoemulsification cataract surgery.2, 9 Over several years, the Royal College of Ophthalmologists have facilitated the defining of a Cataract National Dataset (CND)10 and their 2004 Cataract Surgery Guidelines11 have been amended to encourage the adoption of this data set for electronic data collection as an integral part of normal clinical care by inclusion of the statement: ‘for purposes of audit a robust method of prospective data collection of the CND, preferably electronically, should be the ideal aimed for in all units’.
Possible benefits of CND use include: detailed local and national audit of cataract surgery, robust data collection for individual surgeons for annual appraisal and possibly revalidation, national monitoring of the delivery and clinical outcomes of cataract surgery in the United Kingdom, research, and facilitation of rational decision making for the commissioning of cataract surgery in the NHS. The CND has been further refined by the Cataract Do Once and Share (DOAS) programme,12 which is a clinical engagement arm of Connecting for Health (previously the National Programme for Information technology or NPfIT) which includes within its remit the development of electronic clinical systems for the NHS. Within the DOAS context, and supported by the Royal College of Ophthalmologists, submissions have been made to the NHS Information Standards Board with a view to the establishment of the CND as an NHS approved data set.
The primary aims of the current survey were to pilot the use of the CND collected by means of Electronic Patient Record (EPR) systems in a multi-centre environment and to demonstrate the ability of the CND to deliver certain of its intended benefits, including detailed preoperative profiling of cataract surgery patients and updating of benchmark standards of care in the NHS and beyond.
Methods
Following on from an earlier pilot National Electronic Cataract Surgery Survey,9 this study comprised a cross-sectional survey of NHS Ophthalmology departments that currently use EPR clinical systems purporting to collect the CND prospectively throughout the cataract care pathway. Through the DOAS programme all current ophthalmology EPR software suppliers and customers in the United Kingdom were contacted and permission requested to extract locally collected CND data from cataract surgery EPR systems. Three companies agreed to extract data from their systems without payment. Data collected with three separate EPR systems were received and assessed (by JMS) for conformity and completeness with the CND. Data from one supplier (Medisoft) was found to be almost identical to the CND with few missing items for key variables. Data derived from the other two systems were either significantly incomplete or not sufficiently similar in structure to the CND to permit its use without substantial re-structuring. No funding was available to pay software companies for extraction or data re-structuring and it was also not possible to undertake such work within the resources of the current project. The final data extraction was from sites using a single EPR system, all participating sites having given consent for anonymised data to be remotely extracted from their local databases. Patient identifiers were completely stripped out and site and clinician were pseudo-anonymised. These processes had to be achieved within the tight timescales of the DOAS project. A Local Ethics Committee confirmed that ethics approval was not required, as this study was an audit and no patients, hospitals or health-care workers were identifiable.
Analysis was restricted to patients undergoing surgery for cataract alone or combined with surgery to reduce astigmatism; those patients undergoing other combined surgical procedures were excluded. The mode of data entry into the EPR varied slightly between sites. At all sites collection of demographic data (age, sex, and ethnicity) was dependent on automatic download from the hospital's patient administration system to the EPR and therefore the completeness of these variables was not under the control of the EPR. When used optimally, preoperative and operative data were entered ‘live’ directly into the EPR as an integral part of the care record. Postoperative data were either entered ‘live’ into the system at the clinic consultation or retrospectively from paper-based care records and returns from community optometrists. The data set for analysis closely resembled the CND.10 In terms of operative complications a combined figure for posterior capsule rupture (PCR) or vitreous loss (VL) or both plus zonule rupture with vitreous loss has been presented to capture all occasions of either ‘PCR or VL or both’ in a single ‘index’ figure. A separate figure for ‘simple’ zonule dialysis (without VL) has been presented to differentiate this as a ‘lesser’ surgical complication since many of these are small and surgically relatively trivial. In this report preoperatively, the ‘best-measured VA’ was the best visual acuity (VA) of the VA with habitual correction and the uncorrected VA (UCVA); and where no result was available for either of these measures pinhole VA was used as a proxy. Visual impairment was defined as the best-measured VA of either the surgical or fellow eyes. Postoperatively, the best-measured VA was the best VA of the best-corrected VA (BCVA) that is with optimal postoperative refraction, UCVA and pinhole VA. In some centres, postoperative data were collected prior to postoperative refraction, which necessitated the inclusion of pinhole VA on such occasions.
Statistical methods
χ2 tests were used to investigate potential differences in the proportion of eyes in different VA groups preoperatively and postoperatively. Fisher's exact testing was used when an expected frequency of a cell in a table was less than 5. As a consequence of anonymising patient data it was not possible to know which operations had been performed on two eyes of a single patient. This precluded any ability to statistically adjust for inter-eye correlations. To compensate we have used a stricter probability level for statistical significance, that is, P<0.01 rather than the more usual P<0.05. Statistical analysis was performed in Excel (Microsoft) and Stata.
Results
Data were extracted on 55 567 cataract operations performed at 12 NHS trusts by 406 surgeons between November 2001 and July 2006. The number and percentage of operations and time period over which data were collected for each Trust site are provided in Table 1. Overall 86.0% of operations were performed between January 2004 and July 2006. Patient's age at the time of each operation was recorded in 100% of cases. The mean age (SD) for all patients was 75.4 (10.4) years, for women 76.1 (10.2) years and for men 74.1 (10.7) years. Gender was recorded in 99.9% (n=55 496) of cases, 62.0% (n=34 406) were female. Data on ethnic origin were collected in 31 984 operations (57.6% of all cases) and are shown with range by site in Table 2. Whether cataract surgery was being performed on the patient's first or second eye was recorded in 49 507 operations (89.1%); of these 58.5% (n=28 942) were performed on first eyes and 41.5% (n=20 565) on second eyes.
Preoperative features
Preoperative VA in the operated eye
Preoperative best-measured VA for the operated eye was available for 55 528 (99.9%) eyes. Both preoperatively best-measured VA and whether it was the patient's first- or second-cataract operation were available for 28 916 first eyes and 20 554 second eyes. Table 3 shows the preoperative best-measured VA for all operated eyes (including range by site), for first and second eyes, for the six VA groups: 6/6 or better, 6/9 or better, 6/12 or better, less than 6/12 to 6/18, less than 6/18 to 6/60, and less than 6/60 to NPL. It should be noted that for best-measured VA the 6/6 or better, 6/9 or better and 6/12 or better categories are cumulative. There was a statistically significant difference (P<0.001) in the proportion of eyes with each level of visual impairment between the first and second eyes, with second eyes having lower levels of visual impairment (better VA) before cataract surgery.
Preoperative VA impairment
Assessing VA impairment (the VA in the better eye) before cataract surgery requires a measure of VA for both eyes and this was available for 52 125 operations (93.8%). Both preoperative visual impairment and whether it was the patient's first- or second-cataract operation were available for 46 441 (83.6%) operations, 27 010 for first eyes and 19 431 for second. Table 4 shows the preoperative VA impairment (including range by site), for all operations, for first-eye cases and for second eyes, for the six VA groups: 6/6 or better, 6/9 or better, 6/12 or better, less than 6/12 to 6/18, less than 6/18 to 6/60, and less than 6/60 to NPL. It should again be noted that for best-measured VA the 6/6 or better, 6/9 or better and 6/12 or better categories are cumulative. χ2 testing was used to investigate differences in the proportion in the six VA groups by first or second eye. There was a statistically significant difference (P<0.001) in the proportion of eyes with each level of visual impairment between the first and second eyes, with patients undergoing second eye surgery having lower levels of VA impairment.
Ocular co-pathology (identified as a reason for a guarded visual prognosis in the operated eye)
The presence or absence of ocular co-pathology considered to be a reason for a guarded visual prognosis in the operated eye was recorded in all cases. No reason for a guarded visual prognosis was identified preoperatively in 39 739 (71.5%) of eyes. The spectrum and percentage of eyes with each co-pathology are shown in Table 5.
Characteristics of surgical procedure
Type of admission
The type of admission was recorded for all operations (100%), with 54 723 (98.5%) performed as a day case and 844 (1.52%) performed as an in-patient.
Anaesthetic technique
Details of the anaesthetic technique were recorded in all but one operation. Overall, 95.5% (n=53 043) of operations were performed using local and topical anaesthetic techniques alone, and 4.54% (n=2523) used general anaesthesia with or without a ‘supplemental’ block.
Surgical technique
The surgical technique was recorded for all 55 567 operations (100%). Phacoemulsification was used in 55 389 cases (99.7%), phacoemulsification was converted to extracapsular surgery in 75 cases (0.13%), planned extracapsular surgery was performed in 97 cases (0.17%) and intracapsular surgery was performed in 6 cases (0.01%).
Grade of surgeon
Data on the grade of 405 of the 406 surgeons who contributed to the study were available for 55 515 cases (99.9%). Table 6 shows details of the number of surgeons in each grade and the median, minimum, maximum number of operations performed by surgeons within each grade.
Surgical complications
Operative complications
A record of the presence or absence of operative complications was recorded in 100% of cases (Table 7). One or more operative complications occurred in 2577 cases (4.64%). PCR or vitreous loss or both (PCR or VL or both) occurred in 1068 cases (1.92%), simple zonule dialysis occurred in 256 cases (0.46%), retained lens fragments (dropped nuclei) occurred in 99 cases (0.18%) and supra-choroidal haemorrhage in 38 cases (0.07%). Note that some eyes may have more than one operative complication recorded.
Postoperative complications
The presence or absence of postoperative complications are given in Table 8. These were recorded in 16 731 (30.1%) cases with a median time to postoperative review of 31 days. Table 9 contrasts the demographic and clinical characteristics of eyes that did and did not have an EPR record of their postoperative follow-up consultation.
VA outcomes
Representativeness
Postoperative ‘best-measured’ VA outcome data were available for 40 758 (73.3%) operations, median follow-up 35 days. These cases are contrasted with those for whom data were not available with regard to age, gender, preoperative VA, ocular co-morbidity and ‘PCR or VL or both’ in Table 9. Statistically significant differences were found for all these variables despite the magnitude of these differences appearing clinically unimportant, a consequence of the large sample size and substantial statistical power of these data. BCVA data were available for 24 404 eyes (43.9%) as indicated in Table 10 where outcomes for BCVA are presented by presence or absence of ocular co-pathology. Comparison with outcomes for best-measured VA (top three rows in Table 11) show that these two measures of acuity gave closely similar results overall and by presence or absence of co-pathology. Statistically significant differences could be detected due to the large power of the data set, although numerically and clinically the observed differences between BCVA and best-measured VA were trivial (robust analysis of similarities and differences will be presented elsewhere).
Together these analyses provided reassurance that those for whom best-measured VA was available could be considered representative and that best-measured VA could be used as a legitimate proxy of best-corrected postoperative VA to maximise the utility of the data set in further analysis.
Postoperative ‘best-measured’ VA
Details of postoperative best-measured VA for 40 758 eyes are presented in Table 11. Overall 91.0% (n=37 096) of eyes achieved 6/12 or better and 45.9% (n=18 698) achieved 6/6 or better. For 30 726 eyes with no ocular co-pathology as a reason for a guarded visual prognosis 94.7% (n=29 083) of eyes achieved 6/12 or better and 51.0% (n=15671) 6/6 or better. For 10 032 eyes with ocular co-pathology, 79.9% (n=8013) achieved 6/12 or better and 30.2% (n=3027) achieved 6/6 or better. The presence of any of the specific co-pathologies listed in Table 11, were statistically and significantly associated (P<0.001) with a reduced postoperative best-measured VA, a result which was mirrored in Table 10 using BCVA.
VA outcomes and age
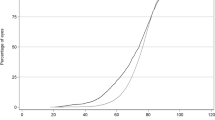
Figure 2 shows the proportion of eyes achieving a postoperative best-measured VA of 6/6 or better, 6/9 or better and 6/12 or better at final follow-up at 5 year age intervals. The figure shows a rapid decline in the percentage of eyes achieving 6/6 or better from the age of 65 onwards, whereas a similar trend is not evident in the percentage of eyes achieving 6/9 or better or 6/12 or better until 80 years.
Change in VA before and after surgery
A cross-tabulation of preoperative vs postoperative best-measured VA is given in Table 12 for 40 724 (73.3%) eyes. This format allows easy access to VA outcomes for each level of preoperative VA. The row percentages indicate the proportion of eyes with a given preoperative VA, which achieved each postoperative VA outcome. Overall 95% of eyes were either the same or better postoperatively.
Discussion
Surgical techniques and cataract extraction rates per head of population have changed enormously over the past two decades. Understanding these changes in the context of patients' clinical presentation and surgical outcomes is essential for quality assurance. Technological advances have not been limited to the procedure itself, the current large scale survey has been made possible by the use of specialty specific EPR systems, which have allowed the authors to assemble a substantial set of detailed data collected as part of routine clinical care. Alongside these developments and facilitated by the Royal College of Ophthalmologists, the DOAS cataract project has further refined a CND.10, 12
Work included assessment of structure, completeness and conformity with the CND of data sets from a number of EPR software sources. It was found that EPR data collected as part of routine clinical care were available in a usable form with a structure sufficiently similar to the CND to permit rapid electronic merger for analysis. With cooperation of relevant stakeholders it has been possible to illustrate ‘proof of concept’ to the NHS Information Standards Board that the CND is essentially ‘fit for purpose’ with large scale data collection and merger feasible provided appropriate local software implementations are in place.
The size of the reported sample is sufficiently large to allow very precise point estimates (percentages and averages) and for detection of small differences between groups and subgroups. Estimates derived from this data set can therefore be expected to be precise provided the sample itself is representative. Available information suggests that overall this is the case, which is reassuring since the participating surgeons and units are at the forefront of cataract EPR implementation and as such may not have been a representative group. The majority of the data (86%) from the 12 participating trusts were collected between January 2004 and July 2006, with no individual surgeon having performed more than 4.6% of the operations and no unit having contributed more than 20% of all operations. In our sample, the mean age was 75.4 years with 62% being females. These basic demographics are essentially identical to national figures for England during this period, for instance during 2004–2005 the mean age for 306 000 reported NHS cataract operations was 75 years with 62% of procedures on female patients. In our sample 98.5% of operations were undertaken as day cases, slightly higher than the figure of 94% nationally.3
EPR programmes are able to force data collection for key variables, thus for preoperative and operative data where collection has been complete or near complete within this data set estimates are likely to be reliable, although for follow-up information the representativeness of the presented results remains less certain. Details of similarities and differences between those with and without follow-up data are provided in Table 9 and relevant results should be interpreted in the appropriate context. Follow-up data for postoperative complications were only available for 30% of eyes. This is due to partial uptake of EPR systems so that preoperative and operative data are fully collected in all centres but this does not always occur for postoperative clinical assessments. In a number of centres VA outcome data were entered independently of postoperative clinical assessments. Best-corrected acuity data were mostly provided by optometrists and were available for 44% with best-measured VA outcome being available for 73% of eyes.
Preoperative acuity in the operated eye was 6/12 or better in 43% of eyes (Table 3). Over the past couple of decades acuity thresholds for listing for surgery have become increasingly lenient, in 1990 under 9% of eyes for surgery had an acuity 6/12 or better,5 by 1997 this had risen to 31%,7 and a report in 2005 on cases carried out between 1998 and 2003 noted this rate to have reached 45% in an 8-centre-pilot electronic audit of over 16 500 cases.9 Although some of these differences may have been due to varying methodology there is a clear trend towards better vision preoperatively over this time period. In this analysis, we chose a definition of best-measured preoperative acuity, which placed an emphasis on habitual correction and unaided vision. Using a different definition of preoperative vision that is the best of habitual, unaided or pinhole-corrected acuity we found 63% of eyes had a preoperative acuity of 6/12 or better. Also of interest in our sample was the observation that listings for first- and second-eye surgery were significantly different with more lenient thresholds being used for second eyes, that is 55% with best-measured 6/12 or better compared with 35% for first eyes (43% overall). In our sample, 23% of eyes had best-measured acuity of 6/9 or better at listing, 17% for first eyes and 34% for second eyes. In terms of VA impairment (best-measured vision in the better eye, Table 4) it is of note that overall 60% of patients had acuity of 6/9 in the better eye, this being 51% for first eyes and 72% for second eyes. With such lenient criteria being applied for listing it would seem advisable for future studies to comment on acuities better than 6/12 preoperatively and postoperatively (as we have done) to avoid a ceiling effect due to scale truncation. In addition there is a case for a robust patient centred quality of life outcome, which is sufficiently brief to be usable in an everyday service delivery environment.
As detailed in Table 7 surgery was uneventful in over 95% of cases, with under 2% of operations being complicated by posterior capsular rupture and/or vitreous loss. This figure included vitreous loss from other causes, including zonule rupture. A composite figure has been presented because the authors are of the view that capsule rupture and/or vitreous loss should trigger an anterior vitrectomy with chamber clearance of all vitreous in the vast majority of cases. This approach avoids temptation to trivialise ‘simple’ PCR without obvious vitreous loss as a lesser complication. Failure to deal adequately with posterior capsular rupture during primary surgery increases the frequency of postoperative problems such as secondary glaucoma, cystoid macular oedema and retinal detachment.13, 14, 15
The halving of this ‘index’ complication, posterior capsular rupture and/or vitreous loss from the 4.4% rate observed in the second national cataract surgery survey of 1997–1998 is noteworthy. In the current survey, 99.7% of operations were by phacoemulsification, which illustrates that UK surgeons have now fully adopted this technique, and the 2% index complication rate reflects what should be expected from a modern surgical service. Just over half of the operations were performed by consultants, approximately 13% by non-consultant career grade surgeons and just over a third by surgical trainees. The 2% index figure, therefore, includes experienced surgeons as well as the most junior surgeons in training. Furthermore, the case mix of this sample was unselected with ‘higher surgical risk’ cases, such as eyes with high myopia, eyes which had previously undergone vitrectomy, eyes with no fundal view and so on included in expected proportions (Table 5).
VA outcomes have been presented separately for BCVA and best-measured VA. Follow-up data for our sample are incomplete and we have presented BCVA on a reduced sample size (n=24 404, 44%) as a ‘gold standard outcome’ as well as best-measured VA (n=40 758, 73%) to optimise available information. Comparison of Tables 10 and 11 confirm that the acuity outcomes were similar for these two measures, both overall and by presence or absence of co-pathology. In view of the larger sample size available for best-measured VA, outcomes for individual co-pathologies have been presented separately in Table 11. Co-pathologies in this case series were recorded only if considered sufficiently severe to be a reason for a guarded prognosis (Table 5). Overall 72% of eyes were free of significant co-pathology, which is less than noted in the second national cataract surgery survey.7, 8 The authors consider the difference in reported rates to be due to different definitions for co-pathology used in the two surveys rather than case mix differences. In this sample, 95% of eyes with no co-pathology and 80% of eyes with co-pathology achieved a ‘best-measured’ VA of 6/12 or better compared with 92 and 77%, respectively, in the second national cataract surgery survey.8 This improvement in outcome may reflect greater experience of phacoemulsification technique which was only used for 77% of operations in the earlier survey compared with close on 100% in this report. It should however also be noted that the preoperative VA was generally better in this sample compared with the earlier sample. The overall 91.4% of eyes which achieved a BCVA of 6/12 or better in this study is similar to the 93% reported recently on a sample of 1000 cases selected for ‘choice’ at a London teaching hospital, in which 16.5% of operations were performed by consultants.16
In Table 12 we have cross-tabulated preoperative acuity and best-measured postoperative acuity. The diagonal shading indicates the ‘line of no change’ with cells to the right and above indicating eyes with a worse postoperative acuity than existed preoperatively and such eyes appear to have been harmed by surgery. While it must be accepted that there will always be a proportion of eyes which are harmed by surgery, where preoperative acuity was good these findings raise questions as to whether the risk to benefit ratio for cataract surgery is being given appropriate consideration in every case.17
The age-related decline in outcome for the more sensitive acuity levels seen in Figure 2 is likely to be contributed to by increasing rates and severity of age-related maculopathy in older patients. Awareness of this decline in expected outcome should be useful to clinicians when counselling elderly patients preoperatively to maintain realistic expectations of their surgery.
Conclusion
This paper illustrates the ability and power of specialty-specific EPR systems to deliver the CND from data collected as part of routine clinical care and provides an opportunity to update modern cataract surgery benchmarks both nationally in the United Kingdom and internationally. Our report details the profile of patients coming forward for cataract surgery, and we describe practice standards for surgical complications and VA outcomes.
References
Courtney P . The national cataract surgery survey: I. Method and descriptive features. Eye 1992; 6: 487–492.
DH. Action on cataracts. Good practice guidance. 2000. http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_4014514.pdf.
DH. Hospital Episode Statistics for England. The information centre for health and social care. http://www.hesonline.nhs.uk/Ease/servlet/ContentServer?siteID=1937&categoryID=193.
Keenan T, Rosen P, Yeates D, Goldacre M . Time trends and geographical variation in cataract surgery rates in England: study of surgical workload. Br J Ophthalmol 2007; 91: 901–904.
Desai P . The national cataract surgery survey: II. Clinical outcomes. Eye 1993; 7: 489–494.
Desai P . The national cataract surgery survey: III. Process features. Eye 1993; 7: 667–671.
Desai P, Reidy A, Minassian DC . Profile of patients presenting for cataract surgery in the UK: national data collection. Br J Ophthalmol 1999; 83: 893–896.
Desai P, Minassian DC, Reidy A . National cataract surgery survey 1997–8: a report of the results of the clinical outcomes. Br J Ophthalmol 1999; 83: 1336–1340.
Johnston RL, Sparrow JM, Canning CR, Tole D, Price NC . Pilot national electronic cataract surgery survey: I. Method, descriptive, and process features. Eye 2005; 19: 788–794.
Service implementation—Do Once and Share. Appendix, P Cataract National Dataset. 2006. http://www.rcophth.ac.uk/docs/college/doas/DOAS_Cataract_Final_Report_Appendix_P.xls.
The Royal College of Ophthalmologists. Cataract surgery guidelines. 2004. http://www.rcophth.ac.uk/docs/publications/published-guidelines/FinalVersionGuidelinesApril2007Updated.pdf.
Service implementation—Do Once and Share. Visual failure (Cataract) Action Team. Final report. 2006. http://www.rcophth.ac.uk/docs/college/doas/DOAS_Cataract_Action_Team_Final_Report_v1.0.DOC.
Chan FM, Mathur R, Ku JJ, Chen C, Chan SP, Yong VS et al. Short-term outcomes in eyes with posterior capsule rupture during cataract surgery. J Cataract Refract Surg 2003; 29: 537–541.
Ray S, D'Amico DJ . Pseudophakic cystoid macular edema. Semin Ophthalmol 2002; 17: 167–180.
Ang GS, Whyte IF . Effect and outcomes of posterior capsule rupture in a district general hospital setting. J Cataract Refract Surg 2006; 32: 623–627.
Zaidi FH, Corbett MC, Burton BJ, Bloom PA . Raising the benchmark for the 21st century—the 1000 cataract operations audit and survey: outcomes, Consultant-supervised training and sourcing NHS choice. Br J Ophthalmol 2007; 91: 731–736.
Sparrow JM . Cataract surgical rates: is there overprovision in certain areas? Br J Ophthalmol 2007; 91: 852–853.
Acknowledgements
We are grateful to all the ophthalmologists who contributed data to this survey, without whose support it would not have been possible. We are also grateful to those software suppliers who submitted data, in particular Medisoft UK whose data formed the basis of these analyses. No funding from any source was provided for data extraction and analysis in this study. Robert Johnston is a Director of Medisoft Limited. Peter Galloway is an advisor to Medisoft in relation to glaucoma but not cataract. This work was presented at the United Kingdom and Ireland Society of Cataract and Refractive Surgeons meeting, September 2006, the European Society of Cataract and Refractive Surgeons meeting, September 2006 and the Royal College of Ophthalmologists Annual Congress, May 2007.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Jaycock, P., Johnston, R., Taylor, H. et al. The Cataract National Dataset electronic multi-centre audit of 55 567 operations: updating benchmark standards of care in the United Kingdom and internationally. Eye 23, 38–49 (2009). https://doi.org/10.1038/sj.eye.6703015
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6703015
Keywords
This article is cited by
-
Non-technical skills simulation-based training model for managing intraoperative posterior capsule rupture during cataract surgery
Eye (2023)
-
A data-driven approach to evaluate factors affecting resident performance in cataract surgery
International Ophthalmology (2023)
-
Evaluation of three biometric devices: ocular parameters and calculated intraocular lens power
Scientific Reports (2022)
-
The cost of laser refractive surgery and supplementary sulcus lens implantation for pseudophakic ametropia and astigmatism, the leeds experience
Eye (2022)
-
Intraocular lens implantation in the absence of capsular support: iris fixation
Eye (2022)