Abstract
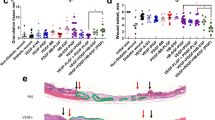
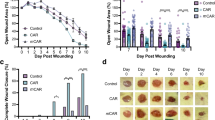
Delivery of therapeutic genes represents an appealing possibility to accelerate healing of wounds that are otherwise difficult to treat, such as those in patients with metabolic disorders or infections. Experimental evidence indicates that in such conditions potentiation of neo-angiogenesis at the wound site might represent an important therapeutic target. Here we explore the efficacy of gene therapy of wound healing with an adeno-associated virus (AAV) vector expressing the 165 amino acid isoform of vascular endothelial growth factor-A (VEGF-A). By gene marker studies, we found that AAV vectors are highly efficient for gene transfer to the rat skin, displaying an exquisite tropism for the panniculus carnosus. Gene expression from these vectors is sustained and persistent over time. Delivery of VEGF165 to full thickness excisional wounds in rats resulted in remarkable induction of new vessel formation, with consequent reduction of the healing time. Histological examination of treated wounds revealed accelerated remodeling of epidermis and dermis, with formation of a thick granular layer, containing numerous newly formed capillaries, as well as vessels of larger size. These data underline the importance of neo-angiogenesis in the healing process and indicate that VEGF gene transfer might represent a novel approach to treat wound healing disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Stadelmann WK, Digenis AG, Tobin GR . Physiology and healing dynamics of chronic cutaneous wounds Am J Surg 1998 176: 26S–38S
Antoniades HN et al. Injury induces in vivo expression of platelet-derived growth factor (PDGF) and PDGF receptor mRNAs in skin epithelial cells and PDGF mRNA in connective tissue fibroblasts Proc Natl Acad Sci USA 1991 88: 565–569
Cromack DT et al. Transforming growth factor beta levels in rat wound chambers J Surg Res 1987 42: 622–628
Kibe Y, Takenaka H, Kishimoto S . Spatial and temporal expression of basic fibroblast growth factor protein during wound healing of rat skin Br J Dermatol 2000 143: 720–727
Hashimoto KR . Regulation of keratinocyte function by growth factors J Dermatol Sci 2000 24: S46–S50
Matsuda H et al. Role of nerve growth factor in cutaneous wound healing: accelerating effects in normal and healing-impaired diabetic mice J Exp Med 1998 187: 297–306
Shukla A, Dubey MP, Srivastava R, Srivastava BS . Differential expression of proteins during healing of cutaneous wounds in experimental normal and chronic models Biochem Biophys Res Commun 1998 244: 434–439
Pierce GF et al. Detection of platelet-derived growth factor (PDGF)-AA in actively healing human wounds treated with recombinant PDGF-BB and absence of PDGF in chronic nonhealing wounds J Clin Invest 1995 96: 1336–1350
Werner S et al. Induction of keratinocyte growth factor expression is reduced and delayed during wound healing in the genetically diabetic mouse J Invest Dermatol 1994 103: 469–473
Brown LF et al. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing J Exp Med 1992 176: 1375–1379
Ansel JC et al. Human keratinocytes are a major source of cutaneous platelet-derived growth factor J Clin Invest 1993 92: 671–678
Nissen NN et al. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing Am J Pathol 1998 152: 1445–1452
Stadelmann WK, Digenis AG, Tobin GR . Impediments to wound healing Am J Surg 1998 176: 39S–47S
Falanga V, Eaglstein WH . The ‘trap’ hypothesis of venous ulceration Lancet 1993 341: 1006–1008
Weckroth M et al. Matrix metalloproteinases, gelatinase and collagenase, in chronic leg ulcers J Invest Dermatol 1996 106: 1119–1124
Altavilla D et al. Inhibition of lipid peroxidation restores impaired vascular endothelial growth factor expression and stimulates wound healing and angiogenesis in the genetically diabetic mouse Diabetes 2001 50: 667–674
Lawrence WT, Diegelmann RF . Growth factors in wound healing Clin Dermatol 1994 12: 157–169
Robson MC, Mustoe TA, Hunt TK . The future of recombinant growth factors in wound healing Am J Surg 1998 176: 80S–82S
Greenhalgh DG . The role of growth factors in wound healing J Trauma 1996 41: 159–167
Greenhalgh DA, Rothnagel JA, Roop DR . Epidermis: an attractive target tissue for gene therapy J Invest Dermatol 1994 103: 63S–69S
Ghazizadeh S, Taichman LB . Virus-mediated gene transfer for cutaneous gene therapy Hum Gene Ther 2000 11: 2247–2251
Vogel JC . Nonviral skin gene therapy Hum Gene Ther 2000 11: 2253–2259
Snyder RO et al. Efficient and stable adeno-associated virus-mediated transduction in the skeletal muscle of adult immunocompetent mice Hum Gene Ther 1997 8: 1891–1900
Su H, Lu R, Kan YW . Adeno-associated viral vector-mediated vascular endothelial growth factor gene transfer induces neovascular formation in ischemic heart Proc Natl Acad Sci USA 2000 97: 13801–13806
Kaplitt MG et al. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain Nat Genet 1994 8: 148–154
Xiao W et al. Adeno-associated virus as a vector for liver-directed gene therapy J Virol 1998 72: 10222–10226
Nakai H et al. Adeno-associated viral vector-mediated gene transfer of human blood coagulation factor IX into mouse liver Blood 1998 91: 4600–4607
Chirmule N et al. Immune responses to adenovirus and adeno-associated virus in humans Gene Therapy 1999 6: 1574–1583
Kay MA et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector Nat Genet 2000 24: 257–261
Monahan PE, Samulski RJ . AAV vectors: is clinical success on the horizon? Gene Therapy 2000 7: 24–30
Descamps V, Blumenfeld N, Beuzard Y, Perricaudet M . Keratinocytes as a target for gene therapy. Sustained production of erythropoietin in mice by human keratinocytes transduced with an adenoassociated virus vector Arch Dermatol 1996 132: 1207–1211
Braun-Falco M, Doenecke A, Smola H, Hallek M . Efficient gene transfer into human keratinocytes with recombinant adeno- associated virus vectors Gene Therapy 1999 6: 432–441
Hengge UR, Mirmohammadsadegh A . Adeno-associated virus expresses transgenes in hair follicles and epidermis Mol Ther 2000 2: 188–194
Donahue BA et al. Selective uptake and sustained expression of AAV vectors following subcutaneous delivery J Gene Med 1999 1: 31–42
Magovern CJ et al. Regional angiogenesis induced in nonischemic tissue by an adenoviral vector expressing vascular endothelial growth factor Hum Gene Ther 1997 8: 215–227
Baumgartner I et al. Constitutive expression of phVEGF165 after intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia Circulation 1998 97: 1114–1123
Arveschoug A, Christensen KS . Constitutive expression of phVEGF165 after intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia Circulation 1999 99: 2967–2968
Griffioen AW, Molema G . Angiogenesis: potentials for pharmacologic intervention in the treatment of cancer, cardiovascular diseases, and chronic inflammation Pharmacol Rev 2000 52: 237–268
Howdieshell TR et al. Antibody neutralization of vascular endothelial growth factor inhibits wound granulation tissue formation J Surg Res 2001 96: 173–182
Laing PT . The development and complications of diabetic foot ulcers Am J Surg 1998 176: 11S–19S
Higley HR, Ksander GA, Gerhardt CO, Falanga V . Extravasation of macromolecules and possible trapping of transforming growth factor-beta in venous ulceration Br J Dermatol 1995 132: 79–85
Trengove NJ et al. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors Wound Repair Regen 1999 7: 442–452
Kessler PD et al. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein Proc Natl Acad Sci USA 1996 93: 14082–14087
Swift ME, Kleinman HK, DiPietro LA . Impaired wound repair and delayed angiogenesis in aged mice Lab Invest 1999 79: 1479–1487
Schmassmann AM . Mechanisms of ulcer healing and effects of nonsteroidal anti-inflammatory drugs Am J Med 1998 104: 43S–51S
Zolotukhin SA et al. A ‘humanized’ green fluorescent protein cDNA adapted for high-level expression in mammalian cells J Virol 1996 70: 4646–4654
Grimm D, Kern A, Rittner K, Kleinschmidt JA . Novel tools for production and purification of recombinant adenoassociated virus vectors Hum Gene Ther 1998 9: 2745–2760
Grimm D, Kern A, Rittner K, Kleinschmidt JA . Novel tools for production and purification of recombinant adenoassociated virus vectors Snyder RO, Xiao X, Samulski J. Production of recombinant adeno-associated viral vectors. In: Dracopoli N et al (eds). Current Protocols in Human Genetics. John Wiley: New York, 1996, pp. 12.11.11–12.12.33
Diviacco S et al. A novel procedure for quantitative polymerase chain reaction by coamplification of competitive templates Gene 1992 122: 313–320
Zentilin L, Marcello A, Giacca M . Involvement of cellular double-strand DNA break-binding proteins in processing of recombinant adeno-associated virus (AAV) genome J Virol 2001 75: 12279–12287
Acknowledgements
This work was supported by grants from MIUR Italy and CNR Italy to MG and from MURST and AIRC to FB. We are grateful to J Kleinschmidt for the pDG plasmid and to B Boziglav and ME Lopez for excellent technical assistance.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Deodato, B., Arsic, N., Zentilin, L. et al. Recombinant AAV vector encoding human VEGF165 enhances wound healing. Gene Ther 9, 777–785 (2002). https://doi.org/10.1038/sj.gt.3301697
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301697
Keywords
This article is cited by
-
Characterization of Viral Genome Encapsidated in Adeno-associated Recombinant Vectors Produced in Yeast Saccharomyces cerevisiae
Molecular Biotechnology (2021)
-
Basic concepts, current evidence, and future potential for gene therapy in managing cutaneous wounds
Biotechnology Letters (2019)
-
Recombinant AAV-mediated in vivo long-term expression and antitumour activity of an anti-ganglioside GM3(Neu5Gc) antibody
Gene Therapy (2015)
-
Topical secretoneurin gene therapy accelerates diabetic wound healing by interaction between heparan-sulfate proteoglycans and basic FGF
Angiogenesis (2014)
-
Adipose-derived Stromal Cells Overexpressing Vascular Endothelial Growth Factor Accelerate Mouse Excisional Wound Healing
Molecular Therapy (2013)