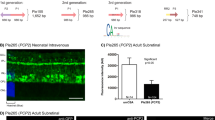
Abstract
Several retinal and choroidal diseases are potentially treatable by intraocular delivery of genes whose products may counter or neutralize abnormal gene expression that occurs as part of the diseases. However, prior to considering a transgene, it is necessary to thoroughly investigate the effects of its expression in normal and diseased eyes. An efficient way to do this is to combine tissue-specific promoters with inducible promoter systems in transgenic mice. In this study, we used this approach to evaluate the effects of ectopic expression of angiopoietin-1 (Ang1) in normal eyes and those with ocular neovascularization. Adult mice with induced expression of Ang1 ubiquitously, or specifically in the retina, appeared normal and had no identifiable changes in retinal or choroidal blood vessels or in retinal function as assessed by electroretinography. Increased expression of Ang1 in eyes with severe retinal ischemia or in eyes with rupture of Bruch's membrane significantly suppressed the development of retinal or choroidal neovascularization, respectively. This inhibition of ocular neovascularization is particularly interesting and noteworthy, because overexpression of Ang1 in skin stimulates neovascularization. Ang1 also significantly reduced VEGF-induced retinal vascular permeability. These data suggest that intraocular delivery of ang1 has potential for treatment of ocular neovascularization and macular edema.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Carmeliet P et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996; 380: 435–439.
Ferrara N et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 1996; 380: 439–442.
Dumont DJ et al. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev 1994; 8: 1897–1909.
Sato TN et al. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature 1995; 376: 70–74.
Davis S et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell 1996; 87: 1161–1169.
Suri C et al. Requisite role of angiopoietin-1, a ligand for the Tie2 receptor, during embryonic angiogenesis. Cell 1996; 87: 1171–1180.
Maisonpierre PC et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 1997; 277: 55–60.
Procopio WN, Pelavin PI, Lee WM, Yeilding NM . Angiopoietin-1 and -2 coiled coil domains mediate distinct homooligomerization patterns, but fibrinogen like domains mediate ligand activity. J Biol Chem 1999: 274.
Koblizek TI et al. Angiopoietin-1 induces sprouting angiogenesis in vitro. Curr Biol 1998; 8: 529–532.
Papapetropoulos A et al. Direct actions of angiopoietin-1 on human endothelium: evidence for network stabalization, cell survival, and interactions with other angiogenic growth factors. Lab Invest 1999; 79: 213–223.
Kwak HJ et al. Angiopoietin-1 is an apoptosis survival factor for endothelial cells. FEBS Lett 1999; 448: 249–253.
Kim I et al. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Circ Res 2000; 86: 24–29.
Witzenbichler B et al. Chemotactic properties of angiopoietin-1 and -2 ligands for the endothelial-specific receptor tyrosine kinase Tie2. J Biol Chem 1998; 273: 18514–18521.
Hayes AJ et al. Angiopoietin-1 and its receptor tie-2 participate in the regulation of capillary-like tubule formation and survival of endothelial cells. Microvasc Res 1999; 58: 224–237.
Kim I et al. Angiopoietin-1 induces endothelial cell sprouting through the activation of focal adhesion kinase and plasmin secretion. Circ Res 2000; 86: 952–959.
Suri C et al. Increased vascularization in mice overexpressing angiopoietin-1. Science 1998; 282: 468–471.
Thurston G et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science 1999; 286: 2511–2515.
Thurston G et al. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med 2000; 6: 460–463.
Ohno-Matsui K et al. Inducible expression of vascular endothelial growth factor in photoreceptors of adult mice causes sever proliferative retinopathy and retinal detachment. Am J Pathol 2002; 160: 711–719.
Takahashi K et al. Intraocular expression of endostatin reduces VEGF-induced retinal vascular permeability, neovascularization, and retinal detachment. FASEB J 2003; 17: 896–898; (Epub 2003 Mar 28).
Okoye G et al. Increased expression of BDNF preserves retinal function and slows cell death from rhodopsin mutation or oxidative damage. J Neuosci 2003; 23: 4164–4172.
Smith LEH et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci 1994; 35: 101–111.
Tobe T et al. Targeted disruption of the FGF2 gene does not prevent choroidal neovascularization in a murine model. Am J Pathol 1998; 153: 1641–1646.
Campochiaro PA, the C99-PKC412-003 Study Group. Reduction of diabetic macular edema by oral administration of the kinase inhibitor PKC412. Invest Ophthalmol Vis Sci 2004; 45:In press.
Derevjanik NL et al. Quantitative assessment of the integrity of the blood–retinalbarrier in mice. Invest Ophthalmol Vis Sci 2002; 43: 2462–2467.
Joussen AM et al. Suppression of diabetic retinopathy with angiopoietin-1. Am J Pathol 2002; 160: 1683–1693.
Okamoto N et al. Transgenic mice with increased expression of vascular endothelial growth factor in the retina: a new model of intraretinal and subretinal neovascularization. Am J Pathol 1997; 151: 281–291.
Chang MA et al. Tetracycline-inducible system for photoreceptor-specific gene expression. Invest Ophthalmol Vis Sci 2000; 41: 4281–4287.
Mitchell V et al. Microwave strategy for improving the simultaneous detection of estrogen and galanin receptor mRNA in the rat hypothalamus. J Histochem Cytochem 2001; 49: 901–910.
Ozaki H et al. Basic fibroblast growth factor is neither necessary nor sufficient for the development of retinal neovascularization. Am J Pathol 1998; 153: 757–765.
Tobe T et al. Evolution of neovascularization in mice with overexpression of vascular endothelial growth factor in photoreceptors. Invest Ophthalmol Vis Sci 1998; 39: 180–188.
Edelman JL, Castro MR . Quantitative image analysis of laser-induced choroidal neovascularization in rat. Exp Eye Res 2000; 71: 523–533.
Acknowledgements
This study was supported by EY05951, EY12609, and core Grant P30EY1765 from the NEI; Research to Prevent Blindness, Inc. (a Lew R Wasserman Merit Award (PAC)); Dr and Mrs William Lake.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nambu, H., Nambu, R., Oshima, Y. et al. Angiopoietin 1 inhibits ocular neovascularization and breakdown of the blood–retinal barrier. Gene Ther 11, 865–873 (2004). https://doi.org/10.1038/sj.gt.3302230
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302230
Keywords
This article is cited by
-
The effect of palm oil-derived tocotrienol-rich fraction in preserving normal retinal vascular diameter in streptozotocin-induced diabetic rats
Graefe's Archive for Clinical and Experimental Ophthalmology (2023)
-
Diabetic retinopathy: emerging concepts of current and potential therapy
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)
-
Targeting the Angiopoietin/Tie Pathway: Prospects for Treatment of Retinal and Respiratory Disorders
Drugs (2021)
-
Inhibition of microRNA-711 limits angiopoietin-1 and Akt changes, tissue damage, and motor dysfunction after contusive spinal cord injury in mice
Cell Death & Disease (2019)
-
Inhibition of integrin α5β1 ameliorates VEGF-induced retinal neovascularization and leakage by suppressing NLRP3 inflammasome signaling in a mouse model
Graefe's Archive for Clinical and Experimental Ophthalmology (2018)