Article Text
Abstract
Background: Neovascular age-related macular degeneration (AMD) has a poor prognosis if left untreated, frequently resulting in legal blindness. Ranibizumab is approved for treating neovascular AMD. However, further guidance is needed to assist ophthalmologists in clinical practice to optimise treatment outcomes.
Methods: An international retina expert panel assessed evidence available from prospective, multicentre studies evaluating different ranibizumab treatment schedules (ANCHOR, MARINA, PIER, SAILOR, SUSTAIN and EXCITE) and a literature search to generate evidence-based and consensus recommendations for treatment indication and assessment, retreatment and monitoring.
Results: Ranibizumab is indicated for choroidal neovascular lesions with active disease, the clinical parameters of which are outlined. Treatment initiation with three consecutive monthly injections, followed by continued monthly injections, has provided the best visual-acuity outcomes in pivotal clinical trials. If continued monthly injections are not feasible after initiation, a flexible strategy appears viable, with monthly monitoring of lesion activity recommended. Initiation regimens of fewer than three injections have not been assessed. Continuous careful monitoring with flexible retreatment may help avoid vision loss recurring. Standardised biomarkers need to be determined.
Conclusion: Evidence-based guidelines will help to optimise treatment outcomes with ranibizumab in neovascular AMD.
Statistics from Altmetric.com
Neovascular age-related macular degeneration (AMD) causes severe and irreversible vision loss, and frequently results in legal blindness, with resulting considerable economic burden.1 2 3 4 5
Pharmacotherapies against vascular endothelial growth factor-A (VEGF-A), a key factor in the pathogenesis of choroidal neovascularisation (CNV), have been introduced to treat neovascular AMD.6 7 8 9 10 Pegaptanib sodium (Macugen, EyeTech, New York), a selective antagonist of the 165 isoform of VEGF-A,11 was approved by the Food and Drug Administration (FDA) in December 2004. Ranibizumab (Lucentis, Novartis Pharma AG, Basel, Switzerland and Genentech, South San Francisco, California), a recombinant, humanised, monoclonal antibody Fab fragment that inhibits all biologically active VEGF-A isoforms, was approved by the FDA in June 2006 (monthly 0.5 mg intravitreal injection).12 13 14 Bevacizumab (Avastin, Genentech), a full-length monoclonal antibody against all VEGF-A isoforms, was approved by the FDA for colorectal cancer in 2004 and later used intravitreally off-label in neovascular AMD.15 16 Head-to-head ranibizumab and bevacizumab trials are under way but are not scheduled to report until 2010 (CATT (NCT00593450), VIBERA (NCT00559715), IVAN and GEFAL trials).
Although preliminary guidelines for anti-VEGF therapies exist,16 17 18 19 20 21 22 more comprehensive clinical practice guidelines on applying ranibizumab are needed to optimise patient outcomes. Ranibizumab Phase III clinical trials in neovascular AMD have studied different treatment schedules, doses and populations, and this review applies the trial evidence to ranibizumab use in clinical practice. We evaluated the licensed 0.5 mg of ranibizumab dose, shown to be more effective than 0.3 mg in pivotal trials,12 13 23 and focused solely on ranibizumab because: pegaptanib showed less visual-acuity (VA) decline than sham injection, although on average treated patients continued to experience vision loss;11 bevacizumab use in neovascular AMD currently remains off-label with relatively few reported clinical trial data and, to date, no completed large, prospective, randomised clinical trials.16
Ranking and sources of evidence
Level I indicates strong evidence (eg, well-designed, randomised, controlled clinical trials that address the issue in question); level II indicates substantial evidence that lacks some qualities (eg, derived from randomised clinical trials but with flaws, such as absent control group or sufficiently long follow-up); level III indicates relatively weak evidence (eg, derived from non-comparative studies without controls, descriptive studies, panel consensus or expert opinion).
A PubMed literature search on 31 October 2008 (restricted to English literature; no date restriction) using the MeSH term macular degeneration (multi) and the words vascular endothelial growth factor, ranibizumab or Lucentis yielded 187 papers. The Cochrane Register of Controlled Trials and the Cochrane Database of Systematic Reviews were also searched, yielding 16 and four references, respectively. A total of 129 relevant articles were selected, from which 74 were selected for detailed assessment. Additional data from abstracts considered relevant to this manuscript were included in the analysis. From this detailed literature search, the primary sources of data were all level I evidence: the Phase III trials MARINA13 and ANCHOR,12 24 including quality-of-life and subgroup analyses,25 26 27 28 and the Phase IIIb trials PIER,23 SAILOR Cohort 1,29 SUSTAIN (assigned level II evidence as only interim data currently available),30 and EXCITE31. A small, open-label study (PrONTO; level III evidence) also provided relevant information,32 and appropriate abstracts covering recent Phase III trial findings (unpublished) were included.
Natural history and assessment of neovascular AMD
What is the natural history or prognosis of untreated neovascular AMD?
A systematic review covering the period 1980 to 2005 assessed studies reporting disease progression outcomes for untreated patients with neovascular age-related macular degeneration (AMD), by using random effects meta-analyses.4 Of 53 studies included, there were 28 randomised clinical trials (RCTs), totalling 4362 patients with untreated neovascular AMD. The most recent RCTs of antivascular endothelial growth factor therapy (VISION,11 MARINA13 and PIER23) were not included. The systematic review found that, on average, one logarithm of the maximum angle of resolution (logMAR) line of visual acuity (VA) was lost by 3 months, three lines by 1 year and four lines by 2 years. This prognosis is relatively similar to that in MARINA, in which sham-treated eyes lost an average of two lines by 1 year and three lines by 2 years and in PIER, in which sham-treated eyes lost an average of three lines by 1 year. In this review, a doubling of the visual angle was found in the first year. At baseline, 20% of eyes already had a VA <20/200, but this proportion rose to 76% by 3 years.4
How should neovascular AMD be diagnosed?
Accurate diagnosis and classification of neovascular AMD using recommended criteria is critical. Assessment should include: history (duration and characteristics of visual symptoms); VA; stereoscopic biomicroscopic slit-lamp fundus examination (78 D or similar lens); fluorescein angiography (FA); and, where possible, optical coherence tomography (OCT).
Logarithm of the minimum angle of resolution (logMAR) VA is preferable to Snellen VA due to its greater sensitivity, ordered progression of letter size (five equally readable letters per line), reproducibility and ability to compare with published trial data.33 The Snellen chart has several limitations such as visual crowding and variable legibility of the letters. Non-geometric letter size progression and a variable number of letters per line also prevent Snellen outcomes from being easily equated to letters or lines of VA change.34 35
For initial diagnosis, FA is deemed mandatory to detect CNV, exclude non-AMD causes (eg, neovascularisation due to myopia, pseudo-xanthoma elasticum, birdshot choroidopathy, etc, which could respond differently to AMD neovascularisation) and determine CNV extent, type, size, location, degree of leakage and proportion of various lesion components.18 36 OCT is also strongly recommended initially to define the extent of retinal thickening and both the localisation and qualitative pattern of extracellular fluid accumulation.37 38 Indocyanine Green (ICG) angiography may also be useful in selected cases, eg, when polypoidal choroidal vasculopathy (PCV)4 39 40 or retinal angiomatous proliferation (RAP)41 42 43 is suspected, or the extent of CNV in occult lesions is unclear.
Ranibizumab therapy for neovascular AMD: indications and contraindications
Which neovascular AMD lesions should be considered for ranibizumab treatment?
All three major CNV subtypes (predominantly classic, occult (with no classic component) and minimally classic) respond to ranibizumab12 13 (table 1). Ranibizumab is primarily indicated for subfoveal (which could also be defined to include juxtafoveal44) lesions with “active disease.”
Recommendations for treatment indication with ranibizumab
The concept of active neovascular AMD is central to these guidelines (level III evidence). A similar concept was proposed in developing guidelines for verteporfin photodynamic therapy (PDT) for AMD and retreatment using specific clinical parameters.45 46 Anti-VEGF therapy specifically targets angiogenesis and vascular permeability,6 47 48 and the active disease concept has evolved to encompass the hallmarks of neovascular disease such as persistent or recurrent extracellular fluid.
The following “starting criteria” (level III evidence) to define active disease may assist in identifying suitable patients for ranibizumab treatment:
abnormal retinal thickness, particularly with evidence of intraretinal, subretinal or subpigment epithelial fluid accumulation, optimally confirmed by OCT;
presence (or recurrence) of intraretinal or subretinal haemorrhage;
new or persistent leakage shown on FA;
CNV enlargement on FA unless solely due to dry, fibrotic staining;
VA deterioration, considered likely to represent CNV activity.
In retrospective analyses of 24-month MARINA study data, ranibizumab was superior to sham across all subgroups based on patient age, gender, CNV lesion type, lesion size, baseline VA and AMD duration.26 VA outcomes were predicted by baseline VA, then CNV lesion size and age (level I evidence). Importantly, for CNV lesion size, smaller lesions had a better prognosis than larger lesions. A subgroup analysis of 12-month ANCHOR study data showed similar results.28
Although clinical data are only available for baseline VA levels of 20/40 (6/12) to 20/320 (6/48), the initial baseline VA was not a limiting factor for response to ranibizumab: all baseline VA subgroups gained with treatment.26 28 For example, cases with active subfoveal/juxtafoveal CNV and VA better than 20/40 should always be considered for treatment, as these have the potential to retain the best possible vision outcomes, particularly for tasks such as reading and driving.
Although the trials did not include cases with the following criteria, no evidence suggests that ranibizumab should be withheld in these populations (level III evidence):17 18
haemorrhage or serous pigment epithelial detachment (PED) involving an area >50% of the entire CNV lesion, particularly if any CNV can be documented before treatment (eg, using ICG);
glaucoma or elevated intraocular pressure;
advanced cataract—cataract surgery should generally follow ranibizumab therapy.
Lesion characteristics such as isolated serous PED without documented CNV,49 RAP or PCV have not been investigated sufficiently in ranibizumab trials. These cases may be considered for ranibizumab therapy, but they might not respond as well, or may respond more slowly,50 than would be expected from the average trial outcomes of other occult lesions. Current trials are investigating some of these subtypes (eg, ranibizumab Phase IV EVEREST PCV trial; clinical trials’ identifier NCT00674323).
What characteristics suggest that ranibizumab would likely be futile?
Based on expert opinion (level III evidence),17 18 and some clinical trial evidence, patients with active disease, but for whom treatment is not generally recommended, were defined by the following criteria:
Structural foveal damage: advanced subretinal fibrosis or significant geographic atrophy involving the foveal centre (both particularly important if longstanding, as any functional benefit from treatment would be unlikely).
Confounding severe ocular disease: vitreous or preretinal haemorrhage obscuring the central macula, or presence of rhegmatogenous retinal detachment (other forms of immediate therapy, eg, vitrectomy, may be indicated before reconsidering ranibizumab).
Retinal pigment epithelial (RPE) tears with subfoveal involvement have been reported to occur occasionally following intravitreal ranibizumab,49 51 52 53 54 55 and may therefore be a relative contraindication. However, to date, no data indicate that continuing ranibizumab in such cases would be deleterious (level III evidence).
Commencing and continuing ranibizumab therapy for neovascular AMD
What are appropriate intervals for the initiation of ranibizumab treatment?
Evidence
Ranibizumab initiation with three consecutive monthly injections appears optimal as this is when the majority of patients experienced most VA gain in all studies (fig 1A–F, tables 2, 3). Improvements occurred rapidly, and the largest VA gain occurred after the first injection. Several studies indicate that untreated subfoveal CNV may grow quickly, on average around 10 μm per day.56 Furthermore, after the first month in the PIER trial, VA deteriorated in the untreated control group by a mean of five letters (one line).23 A recent study reported that delayed initiation of treatment in patients with newly diagnosed AMD was associated with substantial VA loss.57
Mean change from baseline in best-corrected visual acuity by month for (A) MARINA, (B) ANCHOR, (C) PIER, (D) EXCITE, (E) PrONTO, (F) SUSTAIN ((A) Copyright© 2006 Massachusetts Medical Society. All rights reserved; (B) reprinted from Ophthalmology 2009, 116, Brown et al, Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: 2-year results of ANCHOR Study, 57–65, Copyright 2009, with permission from Elsevier; (C) reprinted from Regillo et al, Ranibizumab (Lucentis) in treatment of neovascular age-related macular degeneration (AMD): 2-year results of PIER study, poster PO459 presented at the AAO 2007; (E) reprinted from Am J Ophthalmol 2007, 143, Fung et al, An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration, 566–83, Copyright 2007, with permission from Elsevier).
Summary table of controlled (multicentre, randomised) ranibizumab clinical trials and key efficacy outcomes
Summary table of uncontrolled ranibizumab clinical trials and key efficacy outcomes
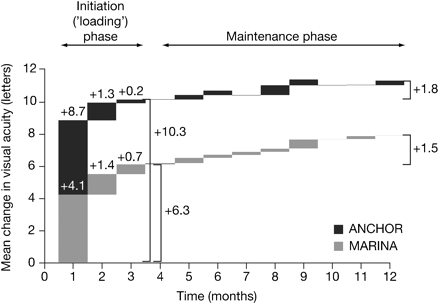
MARINA, ANCHOR12 13 24 and the EXCITE ranibizumab active control arm31 were the only Phase III studies with monthly injections throughout the whole treatment period. Most VA improvement was seen during the initial 3-month phase with subsequent injections appearing to maintain the achieved benefit (fig 2). Prospective clinical trials would be valuable for investigating fewer injections in the initiation phase.
Mean change in visual acuity from baseline (observed cases): difference between each monthly visit for 0.5 mg of ranibizumab in MARINA and ANCHOR (data on file, Novartis Pharma AG, Basel, Switzerland).
Clinical recommendation (level I evidence)
0.5 mg of ranibizumab should be initiated with at least three consecutive monthly intravitreal injections, using an aseptic procedure.58
Treatment should be commenced as soon as possible after diagnosis. As an indication of this time interval, the screening periods permitted before treatment initiation in the clinical studies were ⩽14 or ⩽28 days. Clearly, treatment as early as possible, and at a maximum of within 2 weeks of diagnosis, is ideal. Durations longer than 1 month risk increasing visual loss.23 56
Before administering ranibizumab at months 1 and 2, follow-up examination is recommended: history, VA assessment and slit-lamp fundus examination to identify any ocular side effects or major criteria for treatment failure or discontinuation.
FA is generally recommended only for patients with significant or unexplained vision loss, at the ophthalmologist’s discretion.
OCT detects, localises, classifies and quantifies intraretinal, subretinal and sub-RPE fluid, and is therefore recommended to identify leakage activity before and particularly during follow-up after antiangiogenic therapy. Several prospective trials have demonstrated resolution of fluid following intravitreal ranibizumab together with VA improvement.32 37 38
What are appropriate intervals during the maintenance phase of ranibizumab?
Evidence
In MARINA and ANCHOR, the VA improvements observed with ranibizumab in the first 3 months were sustained (and some additional improvement was seen) over the full 24-month trial period (fig 1A,B; table 2).12 13 24 Ranibizumab also demonstrated angiographic and morphological responses, with improvements in total CNV area and CNV leakage (FA) and in foveal centre-point thickness (OCT).60 Clinically meaningful improvements in patient-reported vision-related function were observed with 0.5 mg of ranibizumab, compared with progressively reduced function with sham (MARINA)25 and verteporfin PDT (ANCHOR).27 These improvements were maintained over the 24-month study period, paralleling the objective VA improvements and, importantly, occurred with treatment of only one eye.
In the PIER study of three consecutive monthly injections followed by fixed quarterly injections,23 ranibizumab demonstrated a clinically meaningful (three lines or more) benefit in mean VA change from baseline compared with sham at 12 and 24 months (figs 1C, 3; table 2). However, although the mean VA improved from baseline in the first 3 months with ranibizumab, this then declined over the 24-month trial period to an average of −2.2 letters, compared with −21.4 letters with sham (fig 1C). These results suggest that quarterly treatment is, on average, inferior to monthly treatment and that more frequent monitoring is needed.
Mean change in visual acuity from baseline for three subgroups of patients in the PIER trial showing that 40% of initial responders retained their initial visual acuity gain during the maintenance phase, although the quarterly regimen did not permit this for the remaining 60% of initial responders (data on file, Novartis Pharma AG, Basel, Switzerland).
The EXCITE study directly compared the PIER quarterly regimen (0.3 mg and 0.5 mg) against monthly injections (0.3 mg).31 Mean VA gain over baseline was observed for the whole 12-month trial in all groups. At month 12 compared with month 3, the VA gain was slightly decreased with quarterly dosing (by −2.2 and −3.1 letters with 0.3 mg and 0.5 mg of ranibizumab, respectively) but was slightly increased (by +0.9 letters) with monthly 0.3 mg of ranibizumab (Figs 1d, 4; table 2).
Mean change in visual acuity from baseline at the end of the loading phase (•) and at 12 months (arrowhead) against the number of injections during 9 months of the maintenance phase (ranibizumab 0.5 mg data unless indicated).
The small, open-label, prospective, single-centre, non-randomised, investigator-sponsored PrONTO study assessed three consecutive monthly injections followed by OCT-guided variable dosing (at ⩾1 month intervals).32 Retreatment criteria were: five-letter loss in the presence of fluid at the macula detected by optical coherence tomography (OCT); ⩾100 μm increase in central retinal thickness (CRT); new-onset classic choroidal neovascularisation (CNV); new macular haemorrhage; or persistent macular fluid detected by OCT. While similar VA outcomes to the MARINA and ANCHOR trials were demonstrated but with fewer intravitreal injections (figs 1E, 4; tables 2, 3), substantial trial design differences limit comparisons. Although small and open label, this study suggests that flexible OCT-guided retreatment could sustain visual gain with fewer injections.
SAILOR Cohort 1 investigated three consecutive monthly injections followed by quarterly monitoring visits and injections guided by VA (more than five-letter loss from the previous highest VA score) and OCT criteria, if available (>100 μm increase in CRT from the previous lowest measurement).29 Additional visits/injections were possible if required. The mean VA change increased from baseline over the first three injections but then decreased (fig 4; table 3) to a mean gain over baseline of 2.3 letters for both ranibizumab doses, a better result than in PIER, but suboptimal when compared with ANCHOR and MARINA.61 These results indicated that quarterly visits were insufficient to monitor and capture disease progression.
Interim results are available from the SUSTAIN trial of three consecutive monthly injections, then monthly monitoring and additional treatment guided by the following criteria: more than five-letter loss in VA from the previous highest VA score during the first 3 months; or >100 μm increase in CRT from the previous lowest measurement during the first 3 months.30 62 At 12 months, most of the first 3 months’ VA gain was maintained (figs 1F, 4; table 3). Although only an interim analysis of 69 patients, these results suggest that flexible, guided dosing with fewer ranibizumab injections and monthly monitoring can maintain efficacy outcomes. However, some VA loss occurred after month 3, whereas fixed monthly injections resulted in further VA improvement during the maintenance phase.
In summary, ranibizumab monthly intravitreal injections demonstrated the best VA outcomes. Studies with less than five injections in the first 12 months generally showed the weakest efficacy benefits (tables 2, 3; figs 1, 4), although results were variable. PrONTO and SUSTAIN showed that monthly monitoring was required to maintain efficacy benefits, compared with SAILOR Cohort 1, which had mandatory quarterly follow-up visits, although more frequent follow-up was possible and performed for many patients.
Clinical recommendation (level I evidence)
A monthly regimen of ranibizumab intravitreal injection demonstrated the best VA outcomes in the clinical trials.
Clinical recommendation (level III evidence)
When a monthly regimen is not possible, a flexible strategy with monthly monitoring is feasible. Benefits could be less than with monthly treatment.
Frequent monitoring aims to detect active disease from: history, VA assessments, slit-lamp examinations and OCT.
FA is generally not essential at this stage but could be considered, particularly if the retinal examination does not explain recent or progressive VA deterioration (FA may identify recurrent leak or CNV enlargement).
If active disease is present or recurs, additional treatment should be initiated quickly to improve functional outcomes.
If the disease is inactive, retreatment is not necessary.
In both cases, patients should be reviewed at each following month using the same assessments, with treatment administered only if active disease is present.
Continued monthly follow-up (with an injection if required) can be recommended, particularly during the first 12 months, in order to detect active disease.
If the clinical signs remain quiescent for a longer period, extending the follow-up intervals may then be justified.
How frequently is ranibizumab therapy needed after 2 years?
In the HORIZON extension trial of MARINA and ANCHOR, 61% of patients needed some additional treatments in the third year; overall better VA and anatomical outcomes after 2 years predicted a longer time to retreatment in this period. Some loss of VA gain occurred, presumably related to undertreatment in the extension period.63
Is treatment with ranibizumab safe?
In a review of safety data from the 3252 patients in ANCHOR, MARINA, PIER and SAILOR (level I evidence) who received over 28 500 intravitreal ranibizumab injections, ranibizumab was found to have a high benefit–risk ratio for treating neovascular AMD.64 Per-injection rates of presumed endophthalmitis (0.05%) or serious intraocular inflammation (0.03%) were low.
A low incidence of serious ocular adverse events has been demonstrated for 0.5 mg of ranibizumab (table 4). In MARINA and ANCHOR (24-month data), the most common were: presumed endophthalmitis (1.3% in MARINA; 1.4% in ANCHOR) and uveitis (1.3% in MARINA; 0.7% in ANCHOR).12 13 24
Summary of key ocular and non-ocular adverse events (AEs) in ranibizumab clinical trials
In MARINA and ANCHOR, the incidence of systemic adverse events was similar across treatment groups. During the 24-month treatment period, the rates of Antiplatelet Trialists’ Collaboration (APTC)65 arterial thromboembolic events (ATEs), including non-fatal myocardial infarction, non-fatal stroke and death from a vascular or unknown cause, were: 3.8% (sham), 4.6% (0.3 mg of ranibizumab) and 4.6% (0.5 mg of ranibizumab) in MARINA; and 4.2% (verteporfin PDT), 4.4% (0.3 mg of ranibizumab) and 5.0% (0.5 mg of ranibizumab) in ANCHOR.12 13 24 In PIER, a low rate of serious ocular adverse events and no ATEs were observed with ranibizumab (table 4).23
An interim SAILOR safety analysis showed a trend for an increase in the incidence of stroke in the 0.5 mg group. The incidence of stroke in the final analysis was 0.7% (0.3 mg) and 1.2% (0.5 mg), but the numerical difference between the two doses was not statistically significant. Incidence of stroke was higher with pre-existing risk factors, particularly a previous stroke history (2.7% (0.3 mg) and 9.6% (0.5 mg)) or arrhythmia.
AMD has previously been associated with a higher risk of stroke.66 67 68 In a retrospective analysis of 15 771 patients with neovascular AMD and 46 408 matched controls, the incidence of ischaemic stroke was 3.5% and 3.6%, respectively, which increased to 35.1% when there was a history of previous ATEs.66 The observed incidence of stroke with ranibizumab was low in these trials, but needs to be continuously monitored in ongoing postmarketing studies. Nevertheless, the benefit–risk profile should be discussed with individual patients, particularly those with a history of, or risk factors for, stroke.
Discussion
Detailed and focused analysis of Phase III clinical trial evidence has generated evidence-based guidelines for using ranibizumab to manage neovascular AMD (summarised in table 5). These guidelines aim to assist ophthalmologists in clinical practice, improve the quality of medical care and optimise the treatment outcomes and quality of life for patients, and are based on the highest level of evidence available.
Summary of clinical recommendations for ranibizumab treatment of neovascular AMD
Clinical evidence indicates that ranibizumab initiation with three consecutive monthly injections is optimal, providing the greatest VA gain, although three versus fewer injections has not been prospectively evaluated. After the initiation phase, the strongest evidence is for continued monthly treatment. As this is frequently not feasible, a flexible individualised approach may achieve similar outcomes to monthly therapy. This, however, is yet to be verified. The flexible approach requires approximate monthly monitoring to capture signs of active disease and reinitiate treatment without delay. Where possible, monthly evaluation should include OCT, as this may be the most sensitive means of detecting VEGF-induced permeability changes.
OCT is strongly recommended for the management of neovascular AMD20 21 and has been found to be generally reproducible, although recent studies have identified some measurement variability.69 70 The new generation of spectral domain and other high-resolution OCTs may provide more accurate assessments, but these instruments have not yet been validated in the context of anti-VEGF therapy in neovascular AMD.71 72 73 Both quantitative OCT (measurements of increased centre-point thickness using “fast” scanning protocols) and qualitative OCT (anatomical evidence of CNV leakage using “regular” scanning protocols) should be used to define VEGF-induced permeability changes in neovascular AMD. Qualitative OCT signs may be the most useful, as these can help to define specific structural changes resulting from CNV leakage (diffuse retinal oedema, intraretinal cysts, subretinal fluid and subretinal pigment epithelial fluid).21 Both types of scan appear to be interchangeable for the comparison of absolute thickness values.74
An ophthalmologist’s full understanding of the particular circumstances and therapeutic needs of their individual patients remains fundamental to providing care. Some of the monitoring techniques discussed within these guidelines are still under evaluation (eg, OCT). Clinician judgement will, therefore, remain important until their use is more clearly understood. Further work aims to identify potential prognostic markers for response to ranibizumab, including the presence of risk genotypes (eg, complement factor H, LOC387715),63 inflammatory factors (eg, C-reactive protein), or other AMD risk factors (eg, smoking). Only interim SUSTAIN results are currently available, so the final data including all recruited patients are awaited with interest.
Overall, ranibizumab has been well tolerated in clinical trials, with a low incidence of ocular and systemic serious adverse events. Postmarketing studies will evaluate its longer-term safety profile in the spectrum of patients treated in clinical practice. Reassessment of the SAILOR trial findings which suggest a possibly greater risk of subsequent stroke among treated cases with a history of stroke or its risk factors (eg, cardiac arrhythmias) is needed using other studies and cohorts.
There are still many unanswered questions to be resolved by future research. For example, are these guidelines applicable to CNV from myopia and PCV; is ICG needed for Asian patients; and what is the role of combination treatment? How many patients need treatment into the second then the third year after initiation? Do any situations alter the pharmacogenetics of ranibizunab (eg, vitrectomy surgery, glaucoma medications)?
Different studies are under way in these areas, such as the ranibizumab Phase IV EVEREST PCV trial (clinical trials’ identifier NCT00674323) and the Phase II MONT BLANC (NCT00433017) and Phase IIIb DENALI (NCT00436553) trials investigating ranibizumab in combination with verteporfin PDT. The HORIZON Extension Study (NCT00379795) has examined the need for treatment into the third year. Insights from the National Eye Institute-sponsored large randomised controlled trial comparing ranibizumab and bevacizumab (Comparison of AMD Treatment Trials (CATT); clinical trials’ identifier NCT00593450) and other similar trials will also contribute substantially to improved understanding of the clinical use of these agents.
Additional evidence should also be collected on patient preferences relating to AMD treatment, as these are important to incorporate within treatment guidelines. To date, patient views have been studied relating to the deleterious impact AMD has on patients’ quality of life, which is often markedly underestimated by ophthalmologists.75 An improved public awareness of the debilitating natural history of AMD and of the benefits from preventive therapies for early stage disease is needed.
These evidence-based guidelines may evolve with better understanding of ranibizumab clinical use from new trial data and increasing clinical practice experience, and should be updated each year. The impact of these guidelines on quality of care and patient well-being should be monitored in clinical practice. Primary efficacy outcomes from the clinical studies, such as the proportion of patients losing ⩽15 letters, gaining ⩾15 letters or maintaining ⩾20/40 vision, could also be used in clinical practice as key audit indicators. Another key outcome for patients is the maintenance of functional vision to enable continued independence, which could, for example, be monitored based on being able to see well enough to read, to drive or to go out shopping.
Acknowledgments
The authors acknowledge medical writing assistance from E Boning from Complete Medical Communications.
REFERENCES
Footnotes
Funding Medical writing assistance was provided under the direction of the authors and was funded unconditionally by Novartis Pharma AG, Basel, Switzerland.
Competing interests PM has received a consultancy fee from Novartis Pharma AG, Pfizer, Solvay and Allergan. He has also been paid lecture fees/honoraria by Novartis Pharma AG, Pfizer, Solvay and Allergan. J-FK has received a consultancy fee from Novartis Pharma AG, Bayer Schering, Alcon, Pfizer and Thea. PL has received a consultancy fee from NeoVista, Allergan, Novartis Pharma AG and QLT. He has also been paid lecture fees/honoraria by Allergan, Novartis Pharma AG, QLT and Optimedica. He has patents with and/or royalties from Iridex Co. FGH has received a consultancy fee from Alcon, Acucela, Bayer Schering and Novartis Pharma AG. He has also been paid lecture fees/honoraria by Alcon and Novartis Pharma AG. CP has received a consultancy fee and lecture fee/honoraria from Novartis Pharma AG. US-E has received a consultancy fee from Novartis Pharma AG, Alcon and Bayer Healthcare. She has also been paid lecture fees/honoraria by Novartis Pharma AG, Alcon, Bayer Healthcare and Carl Zeiss Meditec. YT has received a consultancy fee from Novartis Pharma AG, Alcon Japan, Bausch & Lomb Japan, Pfizer Japan and Santen. He has also been paid lecture fees/honoraria by Novartis Pharma AG, Alcon Japan, Pfizer Japan and Santen. SW has received a consultancy fee from Novartis Pharma AG. He has also been paid lecture fees/honoraria by Novartis Pharma AG, Pfizer and Allergan.
Provenance and Peer review Not commissioned; externally peer reviewed.