Article Text
Abstract
Objectives To report on retinopathy of prematurity (ROP) screening compliance against a national guideline, factors associated with non-compliance and effect on ROP treatment.
Design National cohort study using operational NHS data from the National Neonatal Research Database (NNRD) for the period 2009–2011.
Setting 161 (94%) neonatal units in England.
Population Infants born below 32 weeks’ gestation and/or with a birth weight below 1501 g.
Main outcome measures ROP screening status (‘on-time’, ‘early’, ‘late’, ‘unknown’) and associated infant and neonatal unit characteristics, ROP treatment.
Results The proportion of infants screened on-time increased over the study period (p<0.001). Of 19 821 eligible infants, 7602 (38.4%) were recorded to have received ROP screening in accordance with the national guideline; 7474 (37.8%) received screening outside the recommended time period; data were missing for 4745 (16.7%) infants. For 16 411 infants in neonatal care during the recommended screening period, late screening was significantly associated with lower gestational age (relative risk ratio (RRR) (95% credible interval) for late versus on-time screening 0.83 (0.80 to 0.86) for each increased week of gestation) and care in a neonatal unit providing less than 500 days of intensive care per annum (2.48 (0.99 to 4.99)). Infants screened late were almost 40% more likely to receive ROP treatment (OR (95% CI) 1.36 (1.05 to 1.76)).
Conclusions Understanding organisational differences between neonatal units may help improve ROP screening. Patient-level electronic NHS clinical data offer opportunity for future rapid, low cost, population-based evaluations but require improved data entry.
- Retinopathy of prematurity
- Preterm infants
- Vision screening
- Electronic health records
- Practice guidelines
Statistics from Altmetric.com
- Retinopathy of prematurity
- Preterm infants
- Vision screening
- Electronic health records
- Practice guidelines
What is already known
-
Screening for retinopathy of prematurity is crucial to identify disease early and minimise the risk of visual loss.
-
National guidance for retinopathy of prematurity screening was issued in 2005.
What this study adds
-
There is poor compliance with the national schedule for retinopathy of prematurity screening.
-
Neonatal units providing the lowest volume of intensive care are least likely to screen on-time and more likely to have missing data.
Introduction
Retinopathy of prematurity (ROP) resulting from disordered retinal vascular development in preterm infants is a major preventable cause of visual impairment and accounts for around 3% of all childhood vision loss.1 Careful screening and early initiation of treatment can prevent progression of the disease and reduce the risk of visual loss. In the UK ROP Guideline (May 2008), screening is recommended for all infants born<32 weeks’ gestation or with a birth weight <1501 g.2 The recommended period for the first ophthalmic screening examination is between 30+0 and 30+6 postmenstrual weeks for infants born below 27 weeks’ gestation and between 28 and 35 days postnatal age for infants born at or above 27 weeks’ gestation. This is based on evidence that ‘sight-threatening ROP is unlikely to develop prior to 31 weeks postmenstrual age or 4 to 5 weeks postnatal age’.2 The guideline also includes the recommendation that all infants at risk receive their first ROP screening examination before hospital discharge.
Since 2008, an annual audit of ROP screening has been carried out by the UK Royal College of Paediatrics and Child Health as part of the National Neonatal Audit Programme (NNAP), using data from the UK National Neonatal Research Database (NNRD).3 For the NNAP, compliance for the first ROP examination is assessed against an extended 3-week screening window (by including 1 week on either side of the 1-week period recommended in the national guideline). Here, using the same data set, we (i) report compliance of ROP screening in England during the period of 2009–2011 in strict accordance with guideline recommendations, (ii) investigate infant and neonatal unit characteristics associated with non-compliance with the screening schedule and (iii) examine the effect of ROP screening compliance on the risk for ROP treatment.
Methods
Data source
In England, all neonatal units employ electronic patient record systems to document clinical information. Data are recorded in standardised format and include static items captured once per infant (such as gestational age), episodic items captured once per hospital stay (such as admission time), daily items (such as ventilatory support) and items recorded on an ad hoc basis (such as ROP screening results). Data may be input by neonatal doctors, nurses or clerical staff. Permission is received from the Caldicott Guardians of NHS trusts that are members of the UK Neonatal Collaborative for the release of anonymised patient-level data to the Neonatal Data Analysis Unit where they are merged to create the NNRD, and used for service evaluations including the NNAP and other outputs. The NNRD is approved by the UK National Research Ethics Service (10/80803/151).
Data extraction
We identified eligible infants (born <32+0 weeks’ gestation and/or with a birth weight <1501 g) that died or were discharged between 1 January 2009 and 31 December 2011, from 161 neonatal units (representing 94% of neonatal units in England) contributing data to the NNRD. Infants that died prior to the end of the ROP screening period were excluded. We also excluded infants with missing or implausible birth weights (birth weight greater than four SDs from the mean for gestational age, based on the UK1990 growth reference). We extracted data on the postmenstrual and postnatal age at ROP screening examinations, age at discharge or death, discharge destination (home/paediatric ward), and whether the infant had a record of ROP treatment. For infants that were neonatal unit inpatients during the screening period, we extracted the following information for analysis for associations with screening compliance and ROP treatment: gestational age, birth weight, sex, ethnicity (white or non-white), single or multiple birth, receipt of any antenatal steroids, mode of delivery, number of days of intensive care, mechanical ventilation and oxygen therapy received prior to ROP screening period, and whether the infant was transferred between neonatal units during the screening period or the week before.
ROP screening status
We considered the screening period stated in the UK ROP guideline to be the ‘gold-standard’ time frame for ROP screening. Infants were defined as having received ROP screening ‘on-time’ if any ophthalmic examination occurred during the screening period, or screened ‘early’ or ‘late’ if they only received an ophthalmic examination before or after the stipulated period, respectively. Infants discharged from neonatal care prior to the start of the screening period were considered separately and classified as having received screening ‘prior to discharge’ or ‘after discharge’. If no date for ROP screening was entered into the electronic record, the ROP screening status of the infant was ‘unknown’. It was not possible to distinguish between infants who received screening but for whom data were missing and infants for whom screening was not performed. Similarly, we were unable to differentiate between infants who did not receive ROP treatment and those with a missing treatment record.
Neonatal unit characteristics
Neonatal unit characteristics were investigated using first, the self-reported designation of neonatal units (levels 1–3 as defined by the British Association of Perinatal Medicine (BAPM)),4 and second, based on the mean annual number of neonatal intensive care days (BAPM criteria)5 provided by the neonatal unit during the study period (grouped into <500, 500–999, 1000–1999 and ≥2000 days). For each infant, responsibility for ROP screening was assigned to a specific neonatal unit to determine neonatal unit characteristics associated with non-compliance. If an infant received ROP screening in accordance with the guideline, responsibility for the screening was assigned to the neonatal unit performing the first screen during the period. For infants not screened in accordance with the guideline, responsibility for the screening was assigned to the neonatal unit caring for the infant on the last day of the screening period, or the neonatal unit of discharge for infants discharged prior to the end of screening period. This method of assigning responsibility takes into consideration the possibility of infants being transferred or discharged during the screening period.
Statistical analyses
Group differences in infant and neonatal unit characteristics between infants with different screening statuses were compared using F-tests for normally distributed variables, with robust SEs when variances were unequal. Kruskal–Wallis tests were used for non-normal continuous variables and χ2 tests for categorical variables. Post hoc pair-wise comparisons of screening statuses were carried out with Bonferroni correction for multiple testing.
A Bayesian multinomial logistic model was fitted to identify factors associated with screening compliance. Full details of the model are provided in the statistical appendix. Using ‘on-time’ screen as the baseline category, the relative risk ratio (RRR) and 95% credible intervals (95% CrI) are reported for factors associated with each non-compliant outcome (‘late’/‘early’/‘unknown screen’). The RRR is the ratio of the RRs for the outcome and variable in question. Multivariable logistic regression models were used to identify the effect of ROP screening status on ROP treatment after adjusting for confounders identified in the univariable analysis. Results are presented as adjusted ORs with associated 95% CIs and p values. For both analyses, random effect models were used to account for correlation of outcomes for infants in the same neonatal unit (‘clustering’). The intraclass correlation (ICC, the proportion of residual variation due to clustering after adjusting for covariates) was calculated within each outcome.6 We also calculated the correlation between different screening outcomes which indicates, for example, whether neonatal units which were more likely to screen early compared to on-time and were also more likely to screen late after adjusting for covariates. To assess potential bias introduced by missing data on the results, we conducted sensitivity analyses by allocating all infants with ‘unknown’ screening status into the ‘on-time’ screening group as a best-case scenario and then to the ‘late’ screening group as a worst-case scenario. Data extraction was carried out using SAS V.9.2 (SAS Institute, Cary, North Carolina, USA), multinomial models were fitted using MLwiN V.2.26 (Centre for Multilevel Modelling, Bristol) and all other statistical analyses were performed in Stata V.11 (StataCorp, Texas, USA).
Results
During the study period, 21 722 eligible infants were discharged from participating neonatal units or died prior to discharge. We excluded 1662 infants who died before the end of the ROP screening period, 230 infants with missing or implausible birth weights and nine infants with improbable administrative details. There were 19 821 infants included in the final analysis.
Figure 1 shows the classification of ROP screening status of the eligible infants. Overall, 7602 (38%) were screened in accordance with the guideline, that is, ‘on-time’ or before discharge (if discharged prior to the screening period); though 15 076 (76%) received screening at some point. Of the 2886 infants who remained in neonatal care during the screening period and were screened early, 2368 (82%) were screened within 1 week of the recommended screening window, 280 (10%) during the second week and 238 (8%) more than 2 weeks early. Of the 4010 infants who remained in neonatal care during the screening period and were screened late, 389 (10%) were screened after discharge, 1995 (50%) were screened within 1 week, 885 (22%) during the second week and 741 (18%) more than 2 weeks late.
Classification of retinopathy of prematurity screening status. Each percentage is that of the total 19 821 infants.
There were 3410 infants discharged home or to a paediatric ward prior to the screening period; 820 were screened before discharge and 578 were screened after discharge, of whom 257 were screened in the designated screening period for their gestation and birth weight. Screening status was unknown for 59% (2012/3410) of infants discharged prior to the start of the screening period.
To highlight the differences between our study and the NNAP analysis, we note that application of NNAP criteria to our study population would result in 11 965 (60%) infants being classified as having received screening on-time or before neonatal discharge; 967 (5%) were screened after discharge; 518 (3%) to be considered to have had early screening and 1626 (8%) to have had late screening while still in neonatal care.
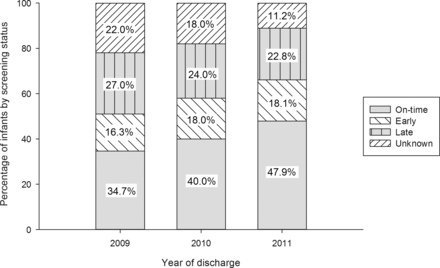
The proportion of infants with unknown screening status decreased over time (figure 2). This was accompanied by an increase in the proportion of infants screened on-time and a corresponding reduction in the proportion of infants screened late (p<0.001 from non-parametric trend test).
Trends over time: percentage in each year with retinopathy of prematurity screening status on-time, early, late or unknown.
Infant and neonatal unit characteristics associated with screening compliance
Table 1 shows the results from the univariable analysis of factors associated with screening compliance. Infants screened late were more preterm, with lower birth weight, and received longer periods of intensive care, mechanical ventilation and oxygen therapy, compared with infants who were screened early or on-time. They were also more likely to be white, to have not received antenatal steroids, to have been born vaginally, and to have been transferred between neonatal units in the week before or during the ROP screening window. Infants screened on-time were more likely to be in higher level, higher intensive care volume neonatal units than those screened early, late or with unknown screening status. Half of the infants with unknown screening status were cared for in neonatal units providing less than 500 intensive care days annually.
Univariable analysis of factors associated with screening compliance for infants in neonatal units during screening period
In the multivariable analysis, lower gestational age was the only infant characteristic associated with late screening (table 2). With each increased week of gestation, the RR of late compared to on-time screening decreased by a mean of 17% (95% CrI (14, 20)). Infants with early or unknown screening status were more likely to be singletons and were born at a higher gestational age. Infants in neonatal units providing the lowest volume of intensive care (<500 days) were more likely to receive screening late compared with infants in neonatal units providing the highest volume of intensive care (≥2000 days) (RRR 2.48 (0.99, 4.99) for late vs on-time). After adjusting for infant characteristics and neonatal unit intensive care volume, there was no pattern of association between the designated neonatal unit level (level 1, 2 or 3) and ROP screening compliance.
Results from Bayesian multinomial logistic model for retinopathy of prematurity (ROP) screening status for infants in neonatal units during screening period
The ICCs (table 2) indicated that infants in the same neonatal unit were likely to have similar screening statuses particularly for unknown screening status (ICC=29%). Neonatal units likely to screen late compared to on-time were also more likely to have missing data (unknown screening status) (correlation r=0.84).
The sensitivity analyses assuming the best-case scenario (all infants with unknown screening assumed to be screened on-time, see online supplementary table S1) showed similar findings to the main analysis. In the worst-case scenario analysis (all infants with unknown screening assumed to be screened late, see online supplementary table S2), there was no apparent association between gestation and late screening (RRR 1.00 (0.98, 1.03)). Associations between late screening and neonatal unit level were different for the main analysis and sensitivity analyses, but as the credible intervals were wide for all analyses the conclusions remained unaltered.
ROP treatment
Of 16 411 infants who were inpatients during their screening period, 383 (2%) were reported to have received ROP treatment; eight of the treated infants were born between 31+0 and 31+6 weeks’ gestations. The proportion of infants treated for ROP was similar over the 3 years (1.9% in 2009, 2.6% in 2010 and 2.5% in 2011); 4.0% of infants screened late required ROP treatment compared with 1.6% of infants who were screened early and 2.3% of those screened on-time. After adjusting for factors found to be associated with ROP treatment from the univariable analysis, infants that were screened late were still significantly more likely to receive ROP treatment (OR 1.36 (1.05, 1.76)) (table 3). We identified no association between early or unknown screening status and ROP treatment.
Results from multivariable logistic regression model showing factors associated with retinopathy of prematurity(ROP) treatment for infants in neonatal units during screening period with complete data, n=16 360
Discussion
This study provides the first comprehensive population analysis of ROP screening and treatment since the reorganisation of neonatal services in England into managed clinical networks in 20037 and the publication of the revised national ROP guideline in 2008.2 ROP screening in strict accordance with national guidelines was received by 38% of eligible infants; though, overall 76% received screening at some point. This was accompanied by an increase in the proportion of infants screened on-time. We found that overall around 2% of infants received ROP treatment, a proportion that showed no statistically significant change over the 3-year study period.
A strength of this study is that specific data collection was not necessary as the analyses were conducted using the NNRD that holds operational electronic clinical data covering admissions to 94% of neonatal units in England. These data are entered during the clinical stay, eliminating recall bias. In a national survey of ROP screening conducted by the Royal College of Paediatrics and Child Health in 1994, only 67% of neonatal units were able to provide infant screening information and this was sought retrospectively.8 In the present study ROP screening information was available on 76% of eligible infants, the proportion of infants with unknown screening status decreased over time and we conducted sensitivity analyses to support our findings. It is possible that ROP treatment rates were underestimated due to missing data. Previous comparative figures are limited; in UK surveys conducted in 19958 and 2005–94 ,9 treatment was estimated at around 5%. A study limitation is that we were unable to validate the reliability of the data. Data quality reports are issued quarterly for the NNAP and as we used the same data set for our analysis, the quality of data are likely to be high. Furthermore, patient discharge summaries are derived from the electronic patient record which would also indicate that reliability is likely to be high.
Almost a quarter of infants were screened late and late screening was more likely in infants born at lower gestations. Though it might be considered plausible that for some infants screening was delayed due to concern that they were not clinically sufficiently stable to tolerate an ophthalmic examination, the number of days of intensive care received prior to the screening period, a marker of illness severity, was not predictive of non-compliance. As the goal of screening is to identify ROP at the prethreshold phase when treatment effectiveness is optimal,10 any delay in screening will add to the risk of missing the treatment window in a rapidly progressing disease. We also found that infants screened late were almost 40% more likely to receive ROP treatment, a conclusion that remained unaltered following adjustment for gestational age and other potential confounders. The reason for this association cannot be inferred from our study but close cooperation between ophthalmic and neonatal teams, with emphasis on careful monitoring and care of the infant during ROP examinations, is likely to help ensure that even the most immature infants are screened on-time.
Variation across neonatal units in compliance with the screening guideline remained after adjusting for infant and neonatal unit characteristics, and after accounting for missing data using sensitivity analyses. Neonatal units providing the lowest volume of intensive care had the highest proportions of infants who were screened late or had missing data. An evaluation of ROP screening in California also found that infants cared for in hospitals with a higher patient volume were less likely to miss screening.11 The reasons for the apparently less efficient delivery of screening in low-volume UK neonatal units, including the adequacy and organisation of current neonatal ophthalmic services, require further investigation.
Unlike a previous report,12 we did not find greater screening non-compliance in infants transferred between neonatal units, suggesting that this may be due to improved delivery of neonatal health services driven by the introduction of managed clinical networks in which clusters of neonatal units assume collective responsibility for care. The annual release by the Royal College of Paediatrics and Child Health of ROP screening compliance in the NNAP may also likely be contributory.
Our data presents an opportunity to review the applicability of the national guideline and can aid future refinements. For example, only 4.1% of infants discharged before the screening period received predischarge screening in accordance with the guideline and around half of the infants who returned to receive postdischarge screening received this in the designated period for their gestation and birth weight. It is possible too that some infants received postdischarge screening but did not have their results documented on the electronic clinical record. Several authors have advocated restriction of the screening criteria to infants born <31 weeks’ gestation or with a birth weight <1251 g, to minimise the number of low-risk infants that would be screened unnecessarily.13–15 This would reduce the number of infants requiring screening in our study by 6385 (32%) infants but eight infants with ROP requiring treatment would have been missed. A case series over a 13-year period conducted in Liverpool also identified 12 infants outside the restricted screening criteria that developed stage 3 ROP.16 Therefore, our findings support the view that ROP screening using the proposed restricted criteria will miss a small group of affected infants. However, the most appropriate strategies to improve the identification of mature at-risk infants and the organisation of screening when this takes place after discharge from the neonatal unit, need further consideration.
In summary, we demonstrate the feasibility of using electronic patient-level information captured in the course of clinical care to evaluate compliance with an important neonatal screening programme. Understanding organisational differences between neonatal units is necessary to improve national performance. Improvements in data completeness and quality will further serve to reap the benefits of this approach.
Acknowledgments
We acknowledge assistance from Neonatal Data Analysis Unit manager Richard Colquhoun, National Neonatal Audit Programme administrators Kim Davis and Glory Oleka and the support of members of the boards of the National Neonatal Audit Programme (Jane Abbott, Roshan Adappa, Lisa Barker, Alan Fenton, Nicola Fitz-Simon, Yvonne Silove, Andrew Wilkinson) and the Neonatal Data Analysis Unit and Medicines for Neonates Programme (Jane Abbott, Deborah Ashby, Peter Brocklehurst, Kate Costeloe, Elizabeth Draper, Michael Goldacre, Jacquie Kemp, Azeem Majeed, Stavros Petrou, Andrew Wilkinson, Alys Young).
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Files in this Data Supplement:
- Data supplement 1 - Online supplement
Footnotes
-
Collaborators Data from the following UK Neonatal Collaborative neonatal networks (neonatal units: lead neonatal clinician) were included in this study: Bedfordshire & Hertfordshire (Bedford Hospital: Dr R Kadalraja; Lister Hospital: Dr J Kefas; Luton & Dunstable Hospital: Dr S Srinnel; Watford General Hospital: Dr C Ramesh). Cheshire & Merseyside (Arrowe Park Hospital: Dr O Rackham; Countess of Chester Hospital: Dr S Brearey; Leighton Hospital: Dr A Thirumurugan; Macclesfield District General Hospital: Dr I Losa; Ormskirk District General Hospital: Dr T McBride; Warrington Hospital: Dr C Zipitis; Whiston Hospital: Dr L Amegavie). Greater Manchester (North Manchester General Hospital: Dr J Moise; Royal Albert Edward Infirmary: Dr C Zipitis; Royal Bolton Hospital: Dr M Yadan; Royal Oldham Hospital: Dr N Maddock; Salford Royal: Dr J Moise; St Mary's Hospital: Dr N Edi-Osagie; Stepping Hill Hospital: Dr C Heal; Tameside General Hospital: Dr J Birch; University Hospital of South Manchester: Dr F Al-Zidgali). Kent & Medway (Darent Valley Hospital: Dr A Hasib; Maidstone, Tunbridge Wells Hospital: Dr H Kisat; Medway Maritime Hospital: Dr A Soe; Queen Elizabeth the Queen Mother Hospital, William Harvey Hospital: Dr D Long). Lancashire & Cumbria (Furness General Hospital, Royal Lancaster Infirmary: Dr J Fedee; Lancashire Women and Newborn Centre, Burnley: Dr M Lama; Royal Preston Hospital: Dr R Gupta; Victoria Hospital: Dr Rawlingson). Midlands Central (George Eliot Hospital: Dr RC de Boer; Kettering General Hospital: Dr P Rao; University Hospital Coventry: Dr K Blake). Midlands North Staffordshire, Shropshire and Black Country (Manor Hospital: Dr AK Bhaduri; New Cross Hospital: Dr C Halahakoon; Royal Shrewsbury Hospital: Dr Deshpande; Russells Hall Hospital: Dr A Mohite; Staffordshire General Hospital: Dr KK Tewary; University Hospital of North Staffordshire: Dr K Palmer). Midlands South West (Alexandra Hospital, Worcestershire Royal Hospital: Dr A Gallagher; Birmingham City Hospital: Dr J Nycyk; Birmingham Heartlands Hospital. Good Hope Hospital: Dr P Simmons; Birmingham Women's Hospital: Dr I Morgen; Hereford County Hospital: Dr HC Underhill). Norfolk Suffolk & Cambridgeshire (Broomfield Hospital: Dr R N Mahesh Babu; Colchester General Hospital: Dr S Dalton; Hinchingbrooke Hospital: Dr H Dixon; Ipswich Hospital: Dr M James; James Paget Hospital: Dr V Jayalal; Norfolk & Norwich University Hospital: Dr M Dyke; Peterborough City Hospital: Dr S Babiker; Princess Alexandra Hospital: Dr T Soe; Queen Elizabeth Hospital: Dr S Rubin; Rosie Maternity Hospital: Dr A Ogilvy-Stuart; West Suffolk Hospital: Dr I Evans). North Central London (Barnet Hospital, Chase Farm Hospital: Dr T Wickham; The Royal Free Hospital: Dr V van Someren; University College Hospital: Dr S Watkin; Whittington Hospital: Dr R Blumberg). North East London (Basildon Hospital: Dr N Sharief; Homerton Hospital: Dr N Aladangady; King George Hospital: Dr B Sharma; Newham General Hospital, The Royal London Hospital, Whipps Cross University Hospital: Dr C Sullivan: North Middlesex University Hospital: Dr L Alsford; Queen’s Hospital: Dr B Sharma: Southend Hospital: Dr A Khan). North Trent (Barnsley District General Hospital: Dr S Hamdan; Bassetlaw District General Hospital, Doncaster Royal Infirmary: Dr JS Ahmed; Chesterfield, North Derbyshire Royal Hospital: Dr A Foo; Rotherham District General Hospital: Dr R Talekar; Scunthorpe General Hospital: Dr P Adiotomre; The Jessop Wing, Sheffield: Dr A Gibson). North West London (Chelsea & Westminster Hospital: Dr M Thomas; Ealing Hospital: Dr R Mathur; Hillingdon Hospital: Dr M Cruwys; Northwick Park Hospital: Dr P Mannix; West Middlesex University Hospital: Dr H Ariff). Northern (Darlington Memorial Hospital, University Hospital of North Durham: Dr M Garbasa; James Cook University Hospital: Dr M Lal; Queen Elizabeth Hospital, Gateshead: Dr D Bosman; Royal Victoria Infirmary: Dr A Fenton; South Tyneside District Hospital: Dr AR Bolton; Sunderland Royal Hospital: Dr M Abu-Harb; University Hospital of North Tees: Dr I Verber; Wansbeck General Hospital: Dr J Olivier). Peninsula (Derriford Hospital: Dr J Larson; North Devon District Hospital: Dr Y Cherinet; Royal Cornwall Hospital: Dr P Munyard; Royal Devon & Exeter Hospital: Dr N Osbourne; Torbay Hospital: Dr M Raman). South East London (Guy's & St Thomas’ Hospital: Dr T Watts; King’s College Hospital: Prof S Hannam; Princess Royal University Hospital, Queen Elizabeth Hospital: Dr S Walter; University Hospital Lewisham: Dr J Kuna). South West London (Croydon University Hospital: Dr YL Chang; Epsom General Hospital, St Helier Hospital: Dr R Shephard; Kingston Hospital: Dr D Lindo; St George’s Hospital: Dr S Calvert). South Central South Coast (North & South) (Basingstoke & North Hampshire Hospital: Dr R Wigfield; Dorset County Hospital: Dr P Wylie; Milton Keynes Foundation Trust Hospital: Dr I Misra; Oxford University Hospitals, Horton Hospital & John Radcliffe Hospital: Dr N Shettihalli; Poole Hospital NHS Foundation Trust: Dr M Khashu; Princess Anne Hospital: Dr M Hall; Queen Alexandra Hospital: Dr C Groves; Royal Berkshire Hospital: Dr P de Halpert; Royal Hampshire County Hospital: Dr D Schapira; Salisbury District Hospital: Dr S Kinsey; St Mary's Hospital: Dr S Butterworth; St Richard's Hospital: Dr A Garg; Stoke Mandeville Hospital: Dr G Whitehead; Wexham Park Hospital: Dr R Sanghavi). Surrey and Sussex (Conquest Hospital, Eastbourne District General Hospital: Dr G Whincup; East Surrey Hospital: Dr K Khader; Frimley Park Hospital: Dr A Mallik; Princess Royal Hospital, Royal Sussex County Hospital: Dr P Amess; Royal Surrey County Hospital: Ms C Godden; St Peter's Hospital: Dr P Reynolds; Worthing Hospital: Dr N Brannan). Trent (King's Mill Hospital: Dr V Noble; Lincoln County Hospital & Pilgrim Hospital: Dr AS Rao; Nottingham City Hospital & Nottingham University Hospital (QMC): Dr S Wardle; Royal Derby Hospital: Dr M Ratnayaka). Western (Gloucestershire Royal Hospital: Dr J Holman; Great Western Hospital: Dr S Zengeya; Royal United Hospital: Dr S Jones; Southmead Hospital: Dr R Wach; St Michael's Hospital: Dr J Tooley; Taunton & Somerset Hospital: Dr RJ Mann; Yeovil District Hospital: Dr M Eaton). Yorkshire (Airedale General Hospital: Dr M Babirecki; Bradford Royal Infirmary: Dr S Seal; Calderdale Royal Hospital: Dr K Schwartz; Dewsbury & District Hospital: Dr D Gibson; Harrogate District Hospital: Dr C Jampala; Hull Royal Infirmary: Dr P Pairaudeau; Leeds Neonatal Service: Dr L Miall; Pinderfield General Hospital: Dr K Shyamannr; Scarborough General Hospital: Dr M Qunib).
-
Contributors HSW analysed the data, drafted and revised the paper. SS wrote the statistical plan, drafted and revised the paper. YS and DG extracted data from the NNRD, cleaned the data and revised the paper. MW revised the draft paper. NM initiated the project, drafted and revised the paper. She is guarantor. Members of the UK Neonatal Collaborative monitored collection of data used in this study.
-
Funding HSW, SS and YS are funded by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research Programme (Grant Reference Number RP-PG-0707-10010) held by NM. DG receives salary support through the Royal College of Paediatrics and Child Health (RCPCH) National Neonatal Audit Programme that is commissioned by the Healthcare Quality Improvement Partnership and funded by the Department of Health. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, the RCPCH or the Department of Health.
-
Competing interests MW is Chair of the National Neonatal Audit Programme; Professor NM is Chair of the Neonatal Data Analysis Unit Steering Board.
-
Ethics approval UK National Research Ethics Service.
-
Provenance and peer review Not commissioned; externally peer reviewed.
Linked Articles
- Fantoms