Article Text
Abstract
Objective Photophobia is an abnormal sensitivity to light experienced by migraineurs and is perhaps caused by cortical hyperexcitability. In clinical studies, an inter-relation between light perception and trigeminal nociception has been demonstrated in migraineurs but not in controls. The purpose of the study was to verify this interaction by functional imaging.
Methods The authors used H2O15 positron emitting tomography (PET) to study the cortical responses of seven migraineurs between attacks and the responses of seven matched control subjects to luminous stimulations at three luminance intensities: 0, 600 and 1800 Cd/m2. All three intensities were both with and without concomitant trigeminal pain stimulation. In order to facilitate habituation, the stimulations were started 30 s before PET acquisitions.
Results When no concomitant pain stimulation was applied, luminous stimulations activated the visual cortex bilaterally in migraineurs (specifically in the cuneus, lingual gyrus and posterior cingulate cortex) but not in controls. Concomitant pain stimulation allowed visual cortex activation in control subjects and potentiated its activation in migraineurs. These activations by luminous stimulations were luminance-intensity-dependent in both groups. Concomitant stimulation by pain was associated with activation of the posterior parietal cortex (BA7) in migraineurs and controls.
Interpretation The study shows the lack of habituation and/or cortical hyperexcitability to light in migraineurs. Moreover, the activation by light of several visual cortex areas (including the primary visual cortex) was potentiated by trigeminal pain, demonstrating multisensory integration in these areas.
- Migraine
- cortical excitability
- photophobia
- trigeminal pain
- positron emitting tomography
- pain simulation
- PET
- functional imaging
Statistics from Altmetric.com
- Migraine
- cortical excitability
- photophobia
- trigeminal pain
- positron emitting tomography
- pain simulation
- PET
- functional imaging
Introduction
Photophobia accompanying migraine headaches is a symptom of important diagnostic value, and has been selected as one of the diagnostic criteria of migraine without aura by the International Headache Society (IHS). Its prevalence is about 85% of patients during attacks,1 2 and it can also be present between attacks.3 Some migraineurs even report bright or flickering lights as a trigger for their attacks,4 and others report photophobia as a signal symptom that announces their attacks. The physiopathology of photophobia has been poorly studied and remains unknown.
Photophobia is defined as ‘hypersensitivity to light’ by the IHS.5 Indeed, some migraineurs report permanent visual discomfort between attacks.6 Electrophysiological studies have led to the formulation of the hypothesis that there is a lack of habituation and/or a cortical hyper-excitability or hyper-responsiveness in migraine sufferers.7 8
Furthermore, there is an inter-relationship between photophobia and pain. The migraine headache is considered as being due to the nociceptive activation of trigeminal cranial afferents from the meninges. A few experimental studies have confirmed an abnormal inter-relationship between light perception and trigeminal nociception in migraineurs but not in controls. Pain stimulation in the ophthalmic territory of the trigeminal nerve decreased tolerance to light,9 while light stimulation lowered the trigeminal nociceptive threshold.10 11
The aim of this study has been to understand better these two aspects of photophobia in migraine: the cortical hyperexcitability and/or lack of habituation, and its inter-relationship with trigeminal pain. In order to achieve this purpose, we used H2O15 positron emitting tomography (PET) to observe cortical responses to visual stimuli in migraineurs and control subjects, both with and without concomitant trigeminal painful stimulation.
Materials and methods
Subjects
Seven female migraineurs ranging in age from 21 to 41 years (four with aura and three without aura, as defined by the IHS diagnosis criteria) were included in our study. All had fewer than six attacks a month (median=2), were always photophobic during attacks, had no other medical history than migraine, had never overused drugs, and had not been under any prophylaxis treatment for at least 1 year. Disease duration ranged from 1 to 34 years (median=18).
We included in our study seven age-matched female control subjects who had no first-order family history of migraine, no personal history of headache (particularly no history of tension-type headache) and no medical history, and were not under any chronic treatment.
Investigators were not blinded to the diagnostic status of the subjects.
To test whether controls or migraineurs between their attacks were photophobic, they were asked to complete a validated visual discomfort auto-questionnaire.12
A cerebral magnetic nuclear resonance imaging (MRI) was performed and interpreted as normal in all subjects, with 3D T1WI gradient echo, T2WI gradient echo, FLAIRWI, diffusion WI, apparent diffusion coefficient cartography (1 T Vision Magnetom, Siemens, Pennsylvania, USA).
A pregnancy test was performed prior to each PET session. The study was approved by the local ethics committee. In accordance with the stipulations of the Helsinki Declaration, informed consent was obtained from each subject before the commencement of our study.
Stimulations
Both the painful and luminous stimulations were continuous and were initiated 30 s before the beginning of each PET scan. Subjects were instructed to keep their eyes open and to avoid blinking during stimulations.
Luminous stimulations were performed through white, half-opaque, polyphane goggles covering the whole visual field, with no fixation point and neither spatial nor chromatic contrast. Three luminance intensities were applied through the goggles' surface: 0 (obscurity), 600 and 1800 Cd/m2. The light was produced by a halogen PAR64 spotlight with a 1000 W CP60 lamp. A transformer fitted with an ammeter allowed precise tuning of luminance intensity. Calibration of the system was verified by using a lux-metre (Minolta LS-100, Japan).
Heat-pain stimulations were performed using a thermode (MSA Thermotest, Somedic AB, Sweden) applied with constant pressure on the face, in the territory of the ophthalmic branch of the right trigeminal nerve (supra-orbital forehead).
In a preliminary session, which was performed at least 3 days from the last migraine attack and 3 days prior to the PET session, the temperature threshold inducing a pain of 30% of the maximum pain perceived was determined. The temperature threshold was determined specifically for each subject and in each lighting condition in order to take into account a modulation by light. The temperature was raised at the rate of 1°C/s, and a constant plateau was maintained for 30 s, at the end of which time subjects were asked to rate their pain between 0 and 100% of maximum pain. Stimulations were performed at different temperatures and were separated by 60 s of rest in obscurity. At rest, the temperature of the thermode was 35°C.
During the PET session, the temperature was increased, rising at the rate of 1°C/s to attain the threshold, inducing a pain of 30% of maximum pain, which had been specifically determined for each subject and in each lighting condition. The plateau was maintained until the end of the acquisition (plateau's duration was 90 s).
PET
The six conditions for migraineurs and control subjects were as follows: no pain and 0 Cd/m2 (NP0), no pain and 600 Cd/m2 (NP600), no pain and 1800 Cd/m2 (NP1800), pain and 0 Cd/m2 (P0), pain and 600 Cd/m2 (P600), pain and 1800 Cd/m2 (P1800). Conditions were randomised and duplicated in two consecutive blocks.
All PET sessions were performed at the same time of day, between 14:00 and 17:00. The PET session began with a 10 min transmission scan at rest with neither painful nor visual stimulation (darkness) during which subjects could adapt to the room's conditions. Scans were started 30 s after the beginning of stimulations. Each acquisition consisted of two 30 s frames scanned by an EXACT HR+ camera (CTI/Siemens, Knoxville, Tennessee, USA) after an intravenous injection of ∼350 MBq of H2O15 into the left arm. They were separated by 7 min of rest during which subjects were told that they could relax. The PET session was performed at least 3 days after the pain threshold preliminary session or migraine attack, and migraineurs were contacted 3 days after the PET session to check whether any further migraine attacks had occurred.
Clinical data were collected at the end of each PET scan: subjects were asked to rate verbally the pain (percentage of maximum pain) and visual discomfort (0=none, 1=light, 2=moderate or 3=severe) experienced during the PET scan.
Statistical analysis
Clinical data were analysed using the Mann–Whitney U test. As we had duplicated the six conditions in two blocks, variation of pain and visual discomfort ratings was defined for each condition as the difference in measure between the first and second block ratings.
PET data were analysed using SPM2 (http://www.fil.ion.ucl.ac.uk/spm) run on a Matlab platform (Mathworks, Natick, Massachusetts, USA). Images were normalised on the stereotaxic MNI (Montreal National Institute) template then smoothed with a 10 mm Gaussian kernel.
First-order analyses of the light effect were performed in a global linear generalised model including the six conditions of the two groups, with proportional scaling normalisation for global cerebral blood flow changes. The following contrasts were studied in each group: NP1800-NP0, NP600-NP0, P1800-P0, P600-P0. The significance of the activations was as follows: p with False Discovery Rate (FDR) correction <0.05 and volume of activation k>300 voxels. The same methodology was used to construct individual models, allowing the extraction of individual first-order contrast for each subject, which were later used for second-order analyses.
Second-order analyses were performed in order to study the effect of light intensity and concomitant pain stimulation. Within-subject analysis of variance (ANOVA) allowed comparisons by SPM2 of the first-order individual contrasts. Two analyses were performed: one in control subjects, the other in migraineurs. Inter-group comparisons were second-order t-test comparisons of the first-order individual contrasts. Each second-order analysis was performed within the luminous-stimulation-related volume; this was the mask of first-order activations by light in the global analysis for the appropriate subjects (p uncorrected <0.05, k>10 000 voxels). The significance level for second-order analyses was as follows: p uncorrected <0.05 and volume of activation k>200 voxels. The same methodology was used for the subgroup comparison of migraineurs with versus without aura.
Labelling of the activations in the Talairach and Tournoux atlas was facilitated by the use of MNI Space Utility SPM2 toolbox (http://www.ihb.spb.ru/∼pet_lab/MSU/MSUMain.html).
Results
Clinical data during PET sessions
The visual discomfort rating variation between the two blocks of duplicated conditions decreased in control subjects (mean=−0.7) while it increased in migraineurs (mean=+0.9). The difference between the two groups was significant (p=0.031).
There was no significant difference between migraineurs and control subjects in pain rating, and no clinical effect of pain on visual discomfort rating was observed.
Activations by light (see table 1 and figures 1, 2)
In control subjects, luminous stimulation did not produce any activation of the visual cortex when no concomitant pain was applied, either at 600 or at 1800 Cd/m2. With concomitant pain, luminous stimulation produced an activation of the primary and secondary visual cortex (BA17 and BA18) and the adjacent posterior cingulate cortex (BA30 and BA31). At 1800 Cd/m2, the activation was larger and also involved the visual associative (BA19) and posterior parietal cortex (BA7).
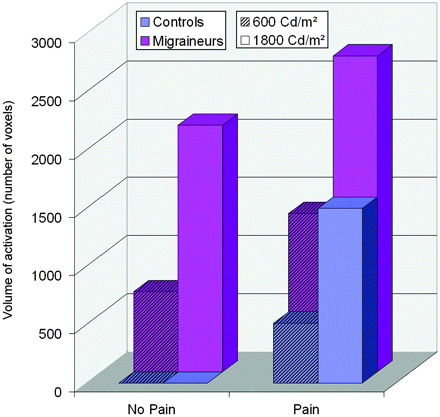
Activations by light
Activations by light. This figure summarises the main results of the study: volumes of activation of visual cortex induced by light in both groups ( migraineurs,
migraineurs,  controls) as a function of pain conditions (No Pain, Pain) in both light intensities
controls) as a function of pain conditions (No Pain, Pain) in both light intensities  600 Cd/m2,
600 Cd/m2,  1800 Cd/m2).
1800 Cd/m2).
Activations by light at 1800 Cd/m2: axial cross-sections at z=0, z=8, z=16 and z=24. Activations were in the cuneus, lingual gyrus, posterior cingulate cortex and precuneus. Note that the volume of activation is greater in migraineurs and that the activation in controls is more rostral.
In migraineurs, visual cortex activation occurred with luminous stimulation even without concomitant pain. The volume of activations increased with increasing luminance intensity from 600 to 1800 Cd/m2. In all conditions, activations involved the primary and secondary visual cortex (BA17 and BA18) and the adjacent posterior cingulate cortex (BA30 and BA31). With concomitant pain, the volume of activations was larger. With concomitant pain at 600 and 1800 Cd/m2, luminous stimulation produced activation in BA7 and BA19.
Z scores were similar in all activations, but for the same statistical threshold, the volume of the activations was larger in migraineurs than in control subjects. Second-order analyses allowed statistical comparison of those different activations.
Intergroup comparison (see table 2)
As expected, when no concomitant pain was applied, activations by light were stronger in migraineurs than in controls in all the areas activated in migraineurs (BA17, BA18, BA30 and BA31).
Intergroup comparisons
At 1800 Cd/m2 and with concomitant pain, activations happened in significantly different areas in migraineurs and control subjects. Luminous-stimulation-related activation was stronger in the secondary visual cortex (BA18) in migraineurs, whereas it was stronger in BA7 and BA19 in control subjects (see figure 3).
Activation of BA7 (precuneus). Controls>migraineurs intergroup comparison of activation by luminous stimulation at 1800 Cd/m2 with concomitant pain stimulation. Second-order analysis within the assumption mask, uncorrected p<0.05, volume k>200 voxels, x=10.
At 600 Cd/m2 and with concomitant pain, no luminous-stimulation-related activation was significantly stronger in migraineurs.
Effect of concomitant pain stimulation (see table 3)
In control subjects, concomitant pain stimulation induced an increase of the luminous-stimulation-related activation by light at 600 and 1800 Cd/m2 in primary and associative visual cortex (BA17, BA18 and BA31). The activation by light of BA7 and BA19 was potentiated by pain only at 1800 Cd/m2.
Potentiation by pain
In migraineurs pain potentiated luminous-stimulation-related activation by light at 600 and 1800 Cd/m2 in BA7 and BA19.
Effect of luminance intensity (see table 4)
Activation by light in the visual cortex was always stronger with 1800 than 600 Cd/m2 stimuli in migraineurs and control subjects. It was not possible to perform this analysis in control subjects when no pain was applied because, in this situation, luminous stimulations did not induce any activation. In addition to the visual cortex, the posterior parietal cortex (BA7) was affected by the effect of luminance intensity in control subjects but not in migraineurs.
Effect of luminance intensity
Other results
Despite the fact that migraineurs had a slight higher visual discomfort auto-questionnaire score than controls (mean 14.9 vs 8.7), the difference between both groups was not significant.
There was no statistically significant difference between the activation by light of migraineurs with aura and migraineurs without aura.
Discussion
Potentiation of visual cortex activation by pain in control subjects
In control subjects, luminous stimulation failed to induce visual cortex activation. This result had been anticipated because of the characteristics of the stimuli applied. All stimuli were continuous, without any contrast, and were started 30 s before the scans. This should have facilitated habituation. Indeed, although our clinical evaluation was very basic (0=none, 1=light, 2=moderate or 3=severe), visual discomfort decreased in control subjects throughout the PET session. This phenomenon could be explained by the physiology of the neurons of the visual system. In the retina, centre/surround interaction in the receptor field of the ganglion cells attenuates light perception when there is no spatial or temporal contrast.13 In the lateral geniculate nuclei, neurons have the same properties.14 In the primary visual cortex, simple and complex cells, which would result from the summation of lateral geniculate nuclei aligned receptive fields,15 16 are sensitive to simple characteristics of their receptive fields such as the spatial or temporal characteristics of oriented gratings, or the colour contrast, but do not respond when those contrasts are absent.17
Concomitant trigeminal pain stimulation induced activation of the visual cortex by light. This interaction may be specific to the ophthalmic territory of trigeminal nerve (V1) nociception. Indeed, clinical studies have shown just such an interaction, since pain stimulation in the V1 territory decreased tolerance to light,10 11 while light stimulation lowered the trigeminal nociceptive threshold.9 This interaction may explain the photophobia in non-migraineurs which occurs when visual pathways and V1 nociception are damaged, as in the case of eye anterior segment diseases,18 19 optic nerve or perichiasmatic tumours,20 subarachnoid haemorrhage and meningitis. Then photophobia may be due to visual cortex overactivation by light.
However, this potentiation of the light-induced visual cortex activation by pain may not be specific to the V1 territory. Indeed, this phenomenon was described recently in the high-level visual cortex area in an object recognition study with concomitant pain stimulation of the hand.21 The authors also observed an intermodal activation of the pregenual anterior cingulate cortex and interpreted their results as a modulation by attentional processes. We could suggest as an hypothesis that, in our study, pain reduced habituation to light through attentional processes.
Effect of luminance intensity on visual cortex activation
Visual cortex activations are usually dependent on stimulus intensity. For example, primary visual cortex responses depend on pattern contrast intensity22 and middle temporal (MT) area responses depend on stimulus velocity.23 24 In our study, activations occurred in response to luminous stimulation in all conditions in migraineurs and only when concomitant pain stimulation was performed in control subjects. These activations appeared to be dependent on the luminance intensity of the stimuli. For methodological reasons, we were unable to test different pain intensities. Further studies are needed to determine whether these luminous-stimulation-related activations, which were pain-stimulation dependent, were modulated by luminous intensity AND pain intensity.
Hyper-responsiveness of the visual cortex in migraineurs
Without concomitant pain, luminous-stimulation-related activations were present in migraineurs only. Migraineurs clearly showed greater luminous-stimulation-related activations than did control subjects. Indeed, these activations were greater in all but one condition (600 Cd/m2 with concomitant pain). It is difficult to interpret this result properly because of the nature of the stimuli we used. As we have seen, these stimuli facilitated habituation. Therefore, it is impossible to know whether the differences between migraineurs and control subjects are due to a lack of habituation or to cortical hyper-reponsiveness. However, our clinical data showed that visual discomfort increased during the study in migraineurs and decreased in controls. This suggests a habituation in controls as opposed to a sensitisation in migraineurs.
Other functional neuroimaging studies of visual cortex responses to light stimulation using functional MRI have yielded conflicting results. The intensity of activation of the visual cortex (measured as BOLD signal amplitude) in migraineurs was higher,25 similar26 or lower27 than in controls. These discrepancies might be due to variations in methodology and in the recruitment of subjects. Functional MRI is based on BOLD signal changes in response to repetitive stimuli. Habituation is a normal phenomenon which occurs as a consequence of the periodicity of the stimuli.28 Differences in activation intensities might in fact be differences in habituation intensities, and might be explained by differences in methodology. Indeed, sensitivity to light differs between migraineurs and is not specific to migraine.29 30 Thus, due to the selection of subjects, sensitivity to light and habituation impairment may have overlapped in both groups and hampered the conclusiveness of the results. One must keep in mind that increased light sensitivity is also present in tension-type headaches, and this type of headache is particularly frequent in the general population.31 Moreover, habituation impairment might also have occurred in control subjects, as it is present in first-order relatives of migraineurs, even though they are not migraineurs themselves.32
In our study, such biases were fully controlled: migraineurs were all photophobic during attacks, and control subjects were highly selected to avoid any overlap between the two groups.
Differential activation of BA7 in migraineurs versus control subjects
In our study, the posterior parietal cortex (precuneus, BA7) was activated by luminous stimulation only when concomitant pain was applied (tables 1, 3). This activation differed in migraineurs and controls. In migraineurs, BA7 was activated at all luminance levels but was not strong enough to be significant when compared with controls (table 2). In control subjects, BA7 activation happened only at 1800 Cd/m2 and was stronger than in migraineurs.
BA7 has been reported as being activated by attentional and working memory tasks, particularly top-down spatial attention.33 34 In our study, there was no such attention task. Moreover, our visual stimuli were purely luminous, without any component of movement, shape or colour. In both groups, we observed that BA7 was activated by luminous stimulations only when concomitant pain was applied. This suggests that pain induced an attentional response to luminous stimuli.
The parallel with psychophysical studies is striking. In those studies, migraineurs had lower performances than control subjects in visual attentional tasks such as the detection of moving dots.35 36 We can assume that the patterns of the stimuli used in those studies may have been responsible for visual discomfort in migraineurs. Some authors have suggested that visual discomfort induced by stimuli may unspecifically capture attention and be responsible for lower performance than in controls.30 37 Our study agrees with this hypothesis, showing a hyper-responsiveness of the visual cortex in relation to a difference in modulation by top-down attentional processes.
This difference between migraineurs and controls in the top-down modulation by BA7 can easily fit in with current models of migraine pathogenesis. It has been shown that motivational salience is mediated by subcortical structures, including locus coeruleus.38 Moreover, locus coeruleus modulates nociception.39 40 Locus coeruleus has diffuse cortical efferences, and its role may be considered as enhancing global neuronal responses to stimuli. But it has been shown recently that it interacts with the visuo-spatial attention network, including the posterior parietal cortex (BA7), to encode the motivational salience.41 Given the role of subcortical structures such as locus coeruleus in migraine pathogenesis,42 we can hypothesise that these structures could be involved in the potentiation by pain of the visual cortex activation, directly or via BA7.
Conclusion
This study shows a stronger activation of the visual cortex in response to luminous stimulation in migraineurs in comparison with the activation elicited from control subjects. Indeed, no visual cortex activation was recorded in control subjects when no concomitant pain was applied. This phenomenon may rely on the habituation induced by the long lasting stimuli we used. Moreover, our results demonstrate a potentiation by pain of visual cortex responses to luminous stimulation in both groups, possibly by attenuating this habituation.
Acknowledgments
We would like to thank the TEP Centre of Toulouse for their participation and encouragement, and the Clinical Investigation Centre for the management of the patients.
References
Footnotes
Funding Délégation Régionale à la Recherche Clinique des Hôpitaux de Toulouse 2005, Centre Hospitalo-Universitaire, 1, avenue du Pr Jean-Poulhès, TSA 50032, 31059 Toulouse cedex, France.
Competing interests None.
Patient consent Obtained.
Ethics approval Ethics approval was provided by the local ethics committee
Provenance and peer review Not commissioned; externally peer reviewed.